Abstract
Background
Methods to improve stratification of small (≤15 mm) lung nodules are needed. We aimed to develop a radiomics model to assist lung cancer diagnosis.
Methods
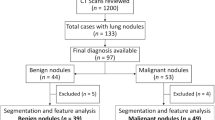
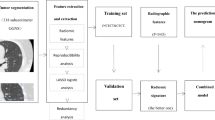
Patients were retrospectively identified using health records from January 2007 to December 2018. The external test set was obtained from the national LIBRA study and a prospective Lung Cancer Screening programme. Radiomics features were extracted from multi-region CT segmentations using TexLab2.0. LASSO regression generated the 5-feature small nodule radiomics-predictive-vector (SN-RPV). K-means clustering was used to split patients into risk groups according to SN-RPV. Model performance was compared to 6 thoracic radiologists. SN-RPV and radiologist risk groups were combined to generate “Safety-Net” and “Early Diagnosis” decision-support tools.
Results
In total, 810 patients with 990 nodules were included. The AUC for malignancy prediction was 0.85 (95% CI: 0.82–0.87), 0.78 (95% CI: 0.70–0.85) and 0.78 (95% CI: 0.59–0.92) for the training, test and external test datasets, respectively. The test set accuracy was 73% (95% CI: 65–81%) and resulted in 66.67% improvements in potentially missed [8/12] or delayed [6/9] cancers, compared to the radiologist with performance closest to the mean of six readers.
Conclusions
SN-RPV may provide net-benefit in terms of earlier cancer diagnosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The radiomics data generated in this study are deposited into the Mendeley database under the accession code https://doi.org/10.17632/rxn95mp24d.1. The R scripts for model development are provided in notebook format at: https://github.com/dr-benjamin-hunter/Small-nodule-radiomics.
References
Gould MK, Tang T, Liu ILA, Lee J, Zheng C, Danforth KN, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192:1208–14.
Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409.
Larici AR, Farchione A, Franchi P, Ciliberto M, Cicchetti G, Calandriello L, et al. Lung nodules: size still matters. Eur Respir Rev. 2017;26:170025.
Baldwin DR, Callister MEJ. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax. 2015;70:794–8.
Lam S, Bryant H, Donahoe L, Domingo A, Earle C, Finley C, et al. Management of screen-detected lung nodules: a Canadian partnership against cancer guidance document. Can J Respir Crit Care Sleep Med. 2020;4:236–65.
Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2013;143:e93S.
Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15:1332–41.
Lung Rads | American College of Radiology. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads.
Zhang R, Tian P, Chen B, Zhou Y, Li W. Predicting lung cancer risk of incidental solid and subsolid pulmonary nodules in different sizes. Cancer Manag Res. 2020;12:8057–66.
Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE, et al. UK Lung Cancer RCT Pilot Screening Trial: Baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax. 2016;71:161–70.
Crosbie PA, Balata H, Evison M, Atack M, Bayliss-Brideaux V, Colligan D, et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax. 2019;74:405–9.
Mascalchi M, Picozzi G, Falchini M, Vella A, Diciotti S, Carrozzi L, et al. Initial LDCT appearance of incident lung cancers in the ITALUNG trial. Eur J Radiol. 2014;83:2080–6.
Kang G, Liu K, Hou B, Zhang N. 3D multi-view convolutional neural networks for lung nodule classification. PLoS ONE. 2017;12:e0188290.
Lyu, J & Ling, SH. Using multi-level convolutional neural network for classification of lung nodules on CT images. in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS vols 2018-July 686-9 (Institute of Electrical and Electronics Engineers Inc., 2018).
Shaffie A, Soliman A, Fraiwan L, Ghazal M, Taher F, Dunlap N, et al. A generalized deep learning-based diagnostic system for early diagnosis of various types of pulmonary nodules. Technol Cancer Res Treat. 2018;17:1533033818798800.
Ardila, D, Kiraly, AP, Bharadwaj, S, Choi, B, Reicher, JJ, Peng, L, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. https://doi.org/10.1038/s41591-019-0447-x (2019).
Massion PP, Antic S, Ather S, Arteta C, Brabec J, Chen H, et al. Assessing the accuracy of a deep learning method to risk stratify indeterminate pulmonary nodules. Am J Respir Crit Care Med. 2020;202:241–9.
Seah J, Tang C, Buchlak QD, Milne MR, Holt X, Ahmad H, et al. Do comprehensive deep learning algorithms suffer from hidden stratification? A retrospective study on pneumothorax detection in chest radiography. BMJ Open. 2021;11:e053024.
Reuben A, Zhang J, Chiou SH, Gittelman RM, Li J, Lee WC, et al. Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat Commun. 2020;11:1–13.
Bartlett EC, Kemp SV, Ridge CA, Desai SR, Mirsadraee S, Morjaria JB, et al. Baseline results of the West London lung cancer screening pilot study—impact of mobile scanners and dual risk model utilisation. Lung Cancer. 2020;148:12–19.
Hunter B, Chen M, Ratnakumar P, Alemu E, Logan A, Linton-Reid K, et al. A radiomics-based decision support tool improves lung cancer diagnosis in combination with the Herder score in large lung nodules. EBioMedicine. 2022;86:104344.
Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas. 2007;1:77–89.
Sun R, Limkin EJ, Vakalopoulou M, Dercle L, Champiat S, Han SR, et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018;19:1180–91.
Beig N, Khorrami M, Alilou M, Prasanna P, Braman N, Orooji M, et al. Perinodular and intranodular radiomic features on lung CT images distinguish adenocarcinomas from granulomas. Radiology. 2019;290:783–92.
Compter I, Verduin M, Shi Z, Woodruff HC, Smeenk RJ, Rozema T, et al. Deciphering the glioblastoma phenotype by computed tomography radiomics. Radiother Oncol. 2021;160:132–9.
Hatt M, Krizsan AK, Rahmim A, Bradshaw TJ, Costa PF, Forgacs A, et al. Joint EANM/SNMMI guideline on radiomics in nuclear medicine. Eur J Nucl Med Mol Imaging. 2022;50:352–75.
Arshad MA, Thornton A, Lu H, Tam H, Wallitt K, Rodgers N, et al. Discovery of pre-therapy 2-deoxy-2- 18 F-fluoro-D-glucose positron emission tomography-based radiomics classifiers of survival outcome in non-small-cell lung cancer patients. Eur J Nucl Med Mol Imaging. 2019;46:455–66.
Lu H, Arshad M, Thornton A, Avesani G, Cunnea P, Curry E, et al. A mathematical-descriptor of tumor-mesoscopic-structure from computed-tomography images annotates prognostic- and molecular-phenotypes of epithelial ovarian cancer. Nat Commun. 2019;10:1–11.
Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, et al. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol. 2011;6:209–17.
Whittaker Brown SA, Padilla M, Mhango G, Powell C, Salvatore M, Henschke C, et al. Interstitial lung abnormalities and lung cancer risk in the national lung screening trial. Chest. 2019;156:1195–203.
Radiotherapy for lung cancer RCR consensus statements.
Baldwin DR, Gustafson J, Pickup L, Arteta C, Novotny P, Declerck J, et al. External validation of a convolutional neural network artificial intelligence tool to predict malignancy in pulmonary nodules. Thorax. 2020;75:306–12.
Binczyk, F, Prazuch, W, Bozek, P & Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. https://doi.org/10.21037/tlcr-20-708 (2021).
Lv W, Wang Y, Zhou C, Yuan M, Pang M, Fang X, et al. Development and validation of a clinically applicable deep learning strategy (HONORS) for pulmonary nodule classification at CT: a retrospective multicentre study. Lung Cancer. 2021;155:78–86.
Ramón y Cajal S, Sesé M, Capdevila C, Aasen T, De Mattos-Arruda L, Diaz-Cano SJ, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med (Berl). 2020;98:161.
Sanduleanu S, Jochems A, Upadhaya T, Even AJG, Leijenaar RTH, Dankers FJWM, et al. Non-invasive imaging prediction of tumor hypoxia: A novel developed and externally validated CT and FDG-PET-based radiomic signatures. Radiother Oncol. 2020;153:97–105.
Jha AK, Mithun S, Jaiswar V, Sherkhane UB, Purandare NC, Prabhash K, et al. Repeatability and reproducibility study of radiomic features on a phantom and human cohort. Sci Rep. 2021;11:1–12.
Yang X, Liu M, Ren Y, Chen H, Yu P, Wang S, et al. Using contrast-enhanced CT and non-contrast-enhanced CT to predict EGFR mutation status in NSCLC patients—a radiomics nomogram analysis. Eur Radiol. 2022;32:2693.
Funding
This manuscript represents independent research funded by: (1) the Royal Marsden Partners Cancer Alliance, (2) the Royal Marsden Cancer Charity, (3) the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research, London, (4) the National Institute for Health Research (NIHR) Biomedical Research Centre at Imperial College, London, (5) Cancer Research UK (C309/A31316). (6) The European Regional Development Fund and Higher Education Funding Council for England.
Author information
Authors and Affiliations
Contributions
BH: Data collection, analysis and interpretation, paper preparation and editing. CA: Data collection, analysis and interpretation, manuscript preparation and editing. MI: Data analysis and interpretation. KL-R: Data analysis. IP, AN, SK, PLS, PLM, CM, TB, EG, JH, AC, SJ, MM and SP: Data collection. HR, JB, CAR, GR, MS and SD: Radiology reads. EOA, RWL and AD: Study design, project supervision, paper feedback and editing.
Corresponding author
Ethics declarations
Competing interests
Professor Devaraj reports personal fees from Brainomix, Roche, and Boehringer Ingelheim and has stock options in Brainomix. Dr Lee is funded by the Royal Marsden NIHR BRC, Royal Marsden Cancer Charity and SBRI (including QURE.AI). RL’s institution receives compensation for time spent in a secondment role for the lung health check programme and as a National Specialty Lead for the National Institute of Health and Care Research. He has received research funding from CRUK, Innovate UK (co-funded by GE Healthcare, Roche and Optellum), SBRI, RM Partners Cancer Alliance and NIHR (co-applicant in grants with Optellum). He has received honoraria from CRUK. The remaining authors declare no competing interests.
Ethics Approval and consent to participate
Health Regulatory Authority (HRA) and Research Ethics Committee (REC) approvals were obtained for the presented study (18/HRA/0434). Informed consent was not required. The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hunter, B., Argyros, C., Inglese, M. et al. Radiomics-based decision support tool assists radiologists in small lung nodule classification and improves lung cancer early diagnosis. Br J Cancer 129, 1949–1955 (2023). https://doi.org/10.1038/s41416-023-02480-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02480-y