Abstract
Background
Resistance to osimertinib in advanced EGFR-mutated non-small cell lung cancer (NSCLC) constitutes a significant challenge for clinicians either in terms of molecular diagnosis and subsequent therapeutic implications.
Methods
This is a prospective single-centre study with the primary objective of characterising resistance mechanisms to osimertinib in advanced EGFR-mutated NSCLC patients treated both in first- and in second-line. Next-Generation Sequencing analysis was conducted on paired tissue biopsies and plasma samples. A concordance analysis between tissue and plasma was performed.
Results
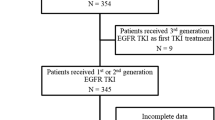
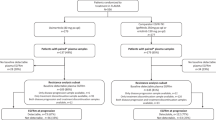
Sixty-five advanced EGFR-mutated NSCLC patients treated with osimertinib in first- (n = 56) or in second-line (n = 9) were included. We managed to perform tissue and liquid biopsies in 65.5% and 89.7% of patients who experienced osimertinib progression, respectively. Acquired resistance mechanisms were identified in 80% of 25 patients with post-progression samples, with MET amplification (n = 8), EGFR C797S (n = 3), and SCLC transformation (n = 2) the most frequently identified. The mean concordance rates between tissue and plasma for the EGFR activating mutation and for the molecular resistance mechanisms were 87.5% and 22.7%, respectively.
Conclusions
Resistance to osimertinib demonstrated to be highly heterogeneous, with MET amplification the main mechanism. Plasma genotyping is a relevant complementary tool which might integrate tissue analysis for the study of resistance mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hendriks LE, Kerr K, Menis J, Mok TS, Nestle U, Passaro A, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:339–57.
Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR -mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378:113–25.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50.
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–37.
Chmielecki J, Gray JE, Cheng Y, Ohe Y, Imamura F, Cho BC, et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun. 2023;14:1–9.
Rolfo C, Mack P, Scagliotti GV, Aggarwal C, Arcila ME, Barlesi F, et al. Liquid biopsy for advanced NSCLC: a consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol. 2021;16:1647–62.
Bonanno L, Dal Maso A, Pavan A, Zulato E, Calvetti L, Pasello G, et al. Liquid biopsy and non-small cell lung cancer: are we looking at the tip of the iceberg? Br J Cancer. 2022;127:383–93.
Piotrowska Z, Ahn M-J, Pang Y-K, How SH, Sang-We K, Voon PJ, et al. 360P ELIOS: A multicentre, molecular profiling study of patients (pts) with epidermal growth factor receptor-mutated (EGFRm) advanced NSCLC treated with first-line (1L) osimertinib. Ann Oncol. 2022;33:S1581–2.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Tavtigian SV, Harrison SM, Boucher KM, Biesecker LG. Fitting a naturally scaled point system to the ACMG/AMP variant classification guidelines. Hum Mutat. 2020;41:1734–7.
Noonan SA, Berry L, Lu X, Gao D, Barón AE, Chesnut P, et al. Identifying the appropriate FISH criteria for defining MET copy number-driven lung adenocarcinoma through oncogene overlap analysis. J Thorac Oncol. 2016;11:1293–304.
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–55.
Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. HER2 amplification and HER2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11:414.
Minari R, Leonetti A, Gnetti L, Zielli T, Ventura L, Bottarelli L, et al. Afatinib therapy in case of EGFR G724S emergence as resistance mechanism to osimertinib. Anticancer Drugs. 2021;32:758–62.
Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49:1693–704.
Lu C, Kang J, Chen H-J, Tu H-Y, Zhou Q, Ye J-Y, et al. Co-occurring alterations in driver genes impact on EGFR-targeted therapy among patients with EGFR-mutant advanced non–small cell lung cancer. Ann Oncol. 2018;29:ix158–9.
Roeper J, Falk M, Chalaris-Rißmann A, Lueers AC, Ramdani H, Wedeken K, et al. TP53 co-mutations in EGFR mutated patients in NSCLC stage IV: A strong predictive factor of ORR, PFS and OS in EGFR mt+ NSCLC. Oncotarget. 2020;11:250.
Labbé C, Cabanero M, Korpanty GJ, Tomasini P, Doherty MK, Mascaux C, et al. Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer. 2017;111:23–9.
Molina-Vila MA, Bertran-Alamillo J, Gascó A, Mayo-de-las-Casas C, Sánchez-Ronco M, Pujantell-Pastor L, et al. Nondisruptive p53 mutations are associated with shorter survival in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2014;20:4647–59.
Choudhury NJ, Marra A, Sui JSY, Flynn J, Yang S-R, Falcon CJ, et al. Molecular biomarkers of disease outcomes and mechanisms of acquired resistance to first-line osimertinib in advanced EGFR-mutant lung cancers. J Thoracic Oncol. 2023;18:463–75.
Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376:629–40.
Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M–positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4:1527–34.
Leonetti A, Minari R, Mazzaschi G, Gnetti L, la Monica S, Alfieri R, et al. Small cell lung cancer transformation as a resistance mechanism to osimertinib in epidermal growth factor receptor-mutated lung adenocarcinoma: case report and literature review. Front Oncol. 2021;11:642190.
van Veggel B, Madeira R Santos JFV, Hashemi SMS, Paats MS, Monkhorst K, Heideman DAM, et al. Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell lung cancer. Lung Cancer. 2020;141:9–13.
Papadimitrakopoulou VA, Wu Y-L, Han J-Y, Ahn M-J, Ramalingam SS, John T, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29:viii741.
Xu C, Wang W, Zhu Y, Yu Z, Zhang H, Wang H, et al. Potential resistance mechanisms using next generation sequencing from Chinese EGFR T790M+ non-small cell lung cancer patients with primary resistance to osimertinib: a multicenter study. Ann Oncol. 2019;30:ii48.
Fanjat Y, Barazzutti H, Di Mauro I, Tabary-Martin L, Duranton-Tanneur V, Gimet S, et al. Molecular follow-up of first-line treatment by osimertinib in lung cancer: importance of using appropriate tools for detecting EGFR resistance mutation C797S. Cancer Genet. 2021;256–257:158–61.
Chen J, Wang J, Wu X. Primary resistance to combination therapy with first- and third-generation EGFR tyrosine kinase inhibitors of lung adenocarcinoma harboring EGFR 19Del/T790M/in trans-C797S mutations with co-occurring CTNNB1 alteration. Onco Targets Ther. 2020;13:6749–53.
Zhao J, Lin G, Zhuo M, Fan Z, Miao L, Chen L, et al. Next-generation sequencing based mutation profiling reveals heterogeneity of clinical response and resistance to osimertinib. Lung Cancer. 2020;141:114–8.
Soria-Comes T, Palomar-Abril V, Ureste MM, Guerola MT, Maiques ICM. Real-world data of the correlation between EGFR determination by liquid biopsy in non-squamous non-small cell lung cancer (NSCLC) and the EGFR profile in tumor biopsy. Pathol Oncol Res. 2020;26:845–51.
Prabhash K, Biswas B, Khurana S, Batra U, Biswas G, Advani SH, et al. CONCORDANCE: a real-world evidence study to evaluate the concordance of detecting epidermal growth factor receptor (EGFR) mutation by circulating tumor DNA* versus tissue biopsy in patients with metastatic non-small cell lung cancer. Indian J Cancer. 2022;59:S11–8.
Choudhury Y, Tan M-H, Shi JL, Tee A, Ngeow KC, Poh J, et al. Complementing tissue testing with plasma mutation profiling improves therapeutic decision-making for patients with lung cancer. Front Med (Lausanne). 2022;9:758464.
Garcia-Pardo M, Czarnecka K, Law JH, Salvarrey A, Fernandes R, Fan J, et al. Plasma-first: accelerating lung cancer diagnosis and molecular profiling through liquid biopsy. Ther Adv Med Oncol. 2022;14:17588359221126152.
Funding
Grant by AIRC (Italian Association for Cancer Research) to TM (grant IG2017-20074).
Author information
Authors and Affiliations
Contributions
MR, AR and TM designed the study. LA, PF, BP, MG, BS and TM enrolled patients and collected the clinical data. CA and FL performed blood sampling. MM, DFM, GD and AL performed tissue sampling. MR, VM, PM, AC, BL, LC, LMS and AR conducted molecular analyses. NR, GL and SEM performed cytological and histological examinations. All the authors contributed to the writing of the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
LA received speakers’ fee for Astra-Zeneca, MSD, Roche, Sanofi and Takeda. LA has been on advisory board for BeiGene, Novartis and Sanofi. TM received speakers’ and consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre, Amgen, Merck, Sanofi. TM received institutional research grants from Astra-Zeneca and Boehringer Ingelheim. AR received institutional grants from Astra-Zeneca.
Ethics approval and consent to participate
The study was approved from the Local Ethic Committee on the 11th of April, 2018 (Protocol 14033, 09/04/2018). Informed consent was obtained prior to any procedure.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leonetti, A., Verzè, M., Minari, R. et al. Resistance to osimertinib in advanced EGFR-mutated NSCLC: a prospective study of molecular genotyping on tissue and liquid biopsies. Br J Cancer 130, 135–142 (2024). https://doi.org/10.1038/s41416-023-02475-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02475-9