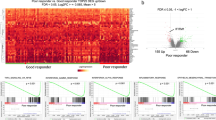
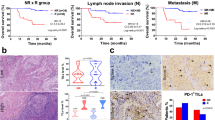
Abstract
Background
Rectal cancer treated with preoperative radiotherapy (RT) provides an interesting model to study changes induced on cancer cell immuno-phenotype that could be exploited by immunotherapy interventions to improve prognosis.
Materials and methods
We assessed the expression of HLA-class-I, β2-microglobulin, TAP1, PD-L1 and STING/IFNβ in preoperative biopsies and respective post-RT surgical specimens from patients with rectal cancer (n = 27). The effect of radiation was further investigated in colorectal adenocarcinoma cell lines HT-29 and Caco-2.
Results
Rectal carcinomas exhibited extensive loss of expression of HLA-Class-I related molecules, which was restored in post-irradiation surgical specimens (P < 0.0001). RT induced the expression of IFNβ and STING in cancer cells and tumour-infiltrating lymphocytes (P < 0.0001). In in vitro experiments, irradiation with 4 Gy or 10 Gy induced the expression of HLA-class-I protein (P < 0.001). PD-L1 levels were transiently induced for two days (P < 0.001). cGAS, STING, IFNβ and the downstream genes (MX1, MX2, UBE2L6v2, IFI6v2 and IFI44) mRNA levels significantly increased after 3 × 8 Gy or 1 × 20 Gy irradiation (P < 0.001). TREX1 mRNA levels remained unaltered.
Conclusions
RT induces the IFN-type-I pathway and the expression of HLA-class-I molecules on rectal carcinoma. The transient induction of PD-L1 expression suggests that long-course daily RT may sustain increased PD-L1 levels. Anti-PD-L1/PD-1 immunotherapy could block this immunosuppressive pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data reported in the study are available for review.
References
Bashford EF, Murray JA, Cramer W. The natural and induced resistance of mice to the growth of cancer. Proc R Soc Lond B Biol Sci. 1907;79:164–87.
Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1:779–86.
Thomas L. Discussion. In: Lawrence HS, editor. Cellular and humoral aspects of the hypersensitive states. New York: Hoeber-Harper; 1959. p. 529–32.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8.
Cruz-Tapias P, Castiblanco J, Anaya JM. Major histocompatibility complex: antigen processing and presentation. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: from bench to bedside. Bogota [Colombia]: El Rosario University Press; 2013. Chapter 10.
Anderson P, Aptsiauri N, Ruiz-Cabello F, Garrido F. HLA class I loss in colorectal cancer: implications for immune escape and immunotherapy. Cell Mol Immunol. 2021;18:556–65.
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharm. 2017;8:561.
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
Koukourakis IM, Tiniakos D, Kouloulias V, Zyogianni A. The molecular basis of immune-radiotherapy. Int J Rad Biol. 2022. https://doi.org/10.1080/09553002.2023.2144960
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52.
Theelen WSME, Chen D, Verma V, Hobbs BP, Peulen HMU, Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467–75.
Fizazi K, Drake CG, Beer TM, Kwon ED, Scher HI, Gerritsen WR, et al. CA184-043, investigators. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020;78:822–30.
Slevin F, Hanna CR, Appelt A, Cunningham C, Marijnen CAM, Sebag-Montefiore D, et al. The long and the short of it: the role of short-course radiotherapy in the neoadjuvant management of rectal cancer. Clin Oncol (R Coll Radiol). 2022;34:e210–17.
Rothkamm K, Christiansen S, Rieckmann T, Horn M, Frenzel T, Brinker A, et al. Radiosensitisation and enhanced tumour growth delay of colorectal cancer cells by sustained treatment with trifluridine/tipiracil and X-rays. Cancer Lett. 2020;493:179–88.
Rodriguez T, Aptsiauri N, Méndez R, Jimenez P, Ruiz-Cabello F, Garrido F. Different mechanisms can lead to the same altered HLA class I phenotype in tumors. Tissue Antigens. 2007;69:259–63.
Koukourakis IM, Giatromanolaki A, Mitrakas A, Koukourakis MI. Loss of HLA-class-I expression in non-small-cell lung cancer: association with prognosis and anaerobic metabolism. Cell Immunol. 2022;373:104495.
Grasso CS, Giannakis M, Wells DK, Hamada T, Mu XJ, Quist M, et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018;8:730–49.
Speetjens FM, de Bruin EC, Morreau H, Zeestraten EC, Putter H, van Krieken JH, et al. Clinical impact of HLA class I expression in rectal cancer. Cancer Immunol Immunother. 2008;57:601–9.
Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RL, Lemmens VE, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174:732–9.
Momburg F, Degener T, Bacchus E, Moldenhauer G, Hämmerling GJ, Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986;37:179–84.
Durrant LG, Ballantyne KC, Armitage NC, Robins RA, Marksman R, Hardcastle JD, et al. Quantitation of MHC antigen expression on colorectal tumours and its association with tumour progression. Br J Cancer. 1987;56:425–32.
Abdel-Wahab Z, Dar MM, Hester D, Vervaert C, Gangavalli R, Barber J, et al. Effect of irradiation on cytokine production, MHC antigen expression, and vaccine potential of interleukin-2 and interferon-gamma gene-modified melanoma cells. Cell Immunol. 1996;171:246–54.
Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004;64:7985–94.
Hareyama M, Imai K, Kubo K, Takahashi H, Koshiba H, Hinoda Y, et al. Effect of radiation on the expression of carcinoembryonic antigen of human gastric adenocarcinoma cells. Cancer. 1991;67:2269–74.
Chapuis AG, Afanasiev OK, Iyer JG, Paulson KG, Parvathaneni U, Hwang JH, et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol Res. 2014;2:27–36.
Sato H, Suzuki Y, Ide M, Katoh T, Noda SE, Ando K, et al. HLA class I expression and its alteration by preoperative hyperthermo-chemoradiotherapy in patients with rectal cancer. PLoS ONE. 2014;9:e108122.
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type I interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–96.
Bursuker I, Pearce MT. Production of interferon-gamma by in vivo tumor-sensitized T cells: association with active antitumor immunity. J Interferon Res. 1990;10:1–11.
Korentzelos D, Wells A, Clark AM. Interferon-γ increases sensitivity to chemotherapy and provides immunotherapy targets in models of metastatic castration-resistant prostate cancer. Sci Rep. 2022;12:6657.
Ito M, Mimura K, Nakajima S, Saito K, Min AKT, Okayama H, et al. Immune escape mechanism behind resistance to anti-PD-1 therapy in gastrointestinal tract metastasis in malignant melanoma patients with multiple metastases. Cancer Immunol Immunother 2002;71:2293–300.
Paulson KG, Voillet V, McAfee MS, Hunter DS, Wagener FD, Perdicchio M, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun. 2018;9:3868.
Aptsiauri N, Carretero R, Garcia-Lora A, Real LM, Cabrera T, Garrido F. Regressing and progressing metastatic lesions: resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol Immunother. 2008;57:1727–33.
Wang X, Schoenhals JE, Li A, Valdecanas DR, Ye H, Zang F, et al. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 2017;77:839–50.
Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618.
Li Y, He M, Zhou Y, Yang C, Wei S, Bian X, et al. The prognostic and clinicopathological roles of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. Front Pharmacol. 2019;10:139.
Hecht M, Büttner-Herold M, Erlenbach-Wünsch K, Haderlein M, Croner R, Grützmann R, et al. PD-L1 is upregulated by radiochemotherapy in rectal adenocarcinoma patients and associated with a favourable prognosis. Eur J Cancer. 2016;65:52–60.
Chen TW, Huang KC, Chiang SF, Chen WT, Ke TW, Chao KSC. Prognostic relevance of programmed cell death-ligand 1 expression and CD8+ TILs in rectal cancer patients before and after neoadjuvant chemoradiotherapy. J Cancer Res Clin Oncol. 2019;145:1043–53.
Chiang SF, Huang CY, Ke TW, Chen TW, Lan YC, You YS, et al. Upregulation of tumor PD-L1 by neoadjuvant chemoradiotherapy (neoCRT) confers improved survival in patients with lymph node metastasis of locally advanced rectal cancers. Cancer Immunol Immunother. 2019;68:283–96.
Tominaga T, Akiyoshi T, Yamamoto N, Taguchi S, Mori S, Nagasaki T, et al. Clinical significance of soluble programmed cell death-1 and soluble programmed cell death-ligand 1 in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS ONE. 2019;14:e0212978.
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Investig. 2014;124:687–95.
Dovedi SJ, Illidge TM. The antitumor immune response generated by fractionated radiation therapy may be limited by tumor cell adaptive resistance and can be circumvented by PD-L1 blockade. Oncoimmunology. 2015;4:e1016709.
Wang QX, Xiao BY, Cheng Y, Wu AW, Zhang T, Wang H, et al. Anti-PD-1-based immunotherapy as curative-intent treatment in dMMR/MSI-H rectal cancer: a multicentre cohort study. Eur J Cancer. 2022;174:176–84.
Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. New Engl J Med. 2022;386:2363–76.
Acknowledgements
The study has been conducted in the context of the ongoing PhD thesis by IMK. AZ, DT and VK are members of the PhD supervising committee.
Funding
The study has been financially supported by the Democritus University of Thrace Special Account, project no 81006. Additional funding was provided by IMK in the context of his ongoing PhD thesis.
Author information
Authors and Affiliations
Contributions
IMK: conception and design, analysis and interpretation of data, immunohistochemical assessment, writing of the first draft and approval of the final draft. EX: in vitro studies analysis and interpretation, drafting the manuscript and approval of the final draft. TIS: flow cytometry experiments, analysis and interpretation, drafting the manuscript and approval of the final draft. MK: immunohistochemical assessment, drafting the manuscript and approval of the final draft. AG: immunohistochemical assessment, analysis and interpretation of the data, drafting the manuscript and approval of the final draft. VK: conception and design, analysis and interpretation of data, critical review of the draft for important intellectual content and approval of the final draft. DT: study supervision, conception and design, analysis and interpretation of the data, critical review of the draft for important intellectual content and approval of the final draft. AZ: study supervision, conception and design, analysis and interpretation of the data, critical review of the draft for important intellectual content and approval of the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Ethics and Research Committee of the Aretaieion University Hospital approved the study (316/26–03–2021), and all patients gave their written informed consent. The study was conducted according to the criteria set by the declaration of Helsinki.
Consent for publication
All patients gave written informed consent to use and publish anonymously all clinical and laboratory data for educational and research purposes.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koukourakis, I.M., Xanthopoulou, E., Sgouras, T.I. et al. Preoperative chemoradiotherapy induces multiple pathways related to anti-tumour immunity in rectal cancer. Br J Cancer 129, 1852–1862 (2023). https://doi.org/10.1038/s41416-023-02459-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02459-9