Abstract
Background
Although nivolumab has a high efficacy, reliable biomarkers are needed to predict the efficacy. We evaluated the nivolumab efficacy according to the TP53 mutation in advanced gastric cancer patients enrolled in the GI-SCREEN project.
Methods
Sequence data of tumour specimens and clinicopathological information of 913 patients with advanced gastric cancer who were enrolled between April 2015 and March 2017 were obtained from the GI-SCREEN database. The follow-up information of 266 patients treated with nivolumab was also provided.
Results
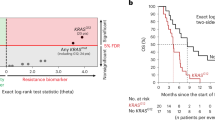
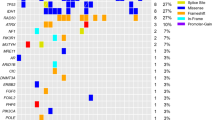
Among 266 patients treated with nivolumab, the objective response rate (ORR) of TP53 wild type (wt) patients (24.6%) was higher than that of TP53 mutant patients (14.8%). Among TP53 mutant patients, the ORR of the frameshift type tended to be higher than the transition and transversion type (23.1%, 13.6%, and 13.0%, respectively). The median progression-free survival (PFS) was statistically longer in TP53 wt patients than in mutant patients (3.3 vs 2.1 months, HR 1.4, 95% CI 1.1–1.9). Among TP53 mutant patients, PFS was statistically longer in the frameshift type than in the transversion type.
Conclusion
Nivolumab showed better efficacy in TP53 wt patients than in mutant patients. Among TP53 mutant patients, the frameshift type may have efficacy from nivolumab treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data generated in this study are not publicly available due to information that could compromise patient privacy or consent but are available upon reasonable request from the corresponding author.
References
Chen LT, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer. 2020;23:510–9.
Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–71.
Janjigian YY, Kawazoe A, Yanez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727–30.
Casak SJ, Marcus L, Fashoyin-Aje L, Mushti SL, Cheng J, Shen YL, et al. FDA approval summary: pembrolizumab for the first-line treatment of patients with MSI-H/dMMR advanced unresectable or metastatic colorectal carcinoma. Clin Cancer Res. 2021;27:4680–4.
Subbiah V, Solit DB, Chan TA, Kurzrock R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) >/=10: a decision centered on empowering patients and their physicians. Ann Oncol. 2020;31:1115–8.
Lei M, Siemers NO, Pandya D, Chang H, Sanchez T, Harbison C, et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab +/- ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. 2021;27:3926–35.
Belyi VA, Ak P, Markert E, Wang H, Hu W, Puzio-Kuter A, et al. The origins and evolution of the p53 family of genes. Cold Spring Harb Perspect Biol. 2010;2:a001198.
Kastenhuber ER, Lowe SW. Putting p53 in context. Cell. 2017;170:1062–78.
Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–58.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10.
Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31.
Baker SJ, Vogelstein B. p53: a tumor suppressor hiding in plain sight. J Mol Cell Biol. 2019;11:536–8.
Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304–17.
Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–13.
Zhang C, Liu J, Xu D, Zhang T, Hu W, Feng Z. Gain-of-function mutant p53 in cancer progression and therapy. J Mol Cell Biol. 2020;12:674–87.
Baugh EH, Ke H, Levine AJ, Bonneau RA, Chan CS. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25:154–60.
Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008.
Hussain SP, Harris CC. p53 mutation spectrum and load: the generation of hypotheses linking the exposure of endogenous or exogenous carcinogens to human cancer. Mutat Res. 1999;428:23–32.
Nakazawa H, English D, Randell PL, Nakazawa K, Martel N, Armstrong BK, et al. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc Natl Acad Sci USA. 1994;91:360–4.
Puisieux A, Ji J, Guillot C, Legros Y, Soussi T, Isselbacher K, et al. p53-mediated cellular response to DNA damage in cells with replicative hepatitis B virus. Proc Natl Acad Sci USA. 1995;92:1342–6.
Takeshima Y, Seyama T, Bennett WP, Akiyama M, Tokuoka S, Inai K, et al. p53 mutations in lung cancers from non-smoking atomic-bomb survivors. Lancet. 1993;342:1520–1.
Leroy B, Anderson M, Soussi T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Hum Mutat. 2014;35:672–88.
Yang RK, Qing Y, Jelloul FZ, Routbort MJ, Wang P, Shaw K, et al. Identification of biomarkers of immune checkpoint blockade efficacy in recurrent or refractory solid tumor malignancies. Oncotarget. 2020;11:600–18.
Cortez MA, Ivan C, Valdecanas D, Wang X, Peltier HJ, Ye Y, et al. PDL1 regulation by p53 via miR-34. J Natl Cancer Inst. 2016;108:djv303.
Nakamura Y, Fujisawa T, Taniguchi H, Bando H, Okamoto W, Tsuchihara K, et al. SCRUM-Japan GI-SCREEN and MONSTAR-SCREEN: path to the realization of biomarker-guided precision oncology in advanced solid tumors. Cancer Sci. 2021;112:4425–32.
Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med. 2020;26:1859–64.
Pan M, Jiang C, Tse P, Achacoso N, Alexeeff S, Solorzano AV, et al. TP53 gain-of-function and non-gain-of-function mutations are differentially associated with sidedness-dependent prognosis in metastatic colorectal cancer. J Clin Oncol. 2022;40:171–9.
Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–8.
Soussi T, Dehouche K, Beroud C. p53 website and analysis of p53 gene mutations in human cancer: forging a link between epidemiology and carcinogenesis. Hum Mutat. 2000;15:105–13.
Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, et al. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas Project. Clin Cancer Res. 2017;23:4441–9.
Liu Y, Sethi NS, Hinoue T, Schneider BG, Cherniack AD, Sanchez-Vega F, et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell. 2018;33:721.e8–35.e8.
Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10.
Egashira A, Morita M, Kakeji Y, Sadanaga N, Oki E, Honbo T, et al. p53 gene mutations in esophageal squamous cell carcinoma and their relevance to etiology and pathogenesis: results in Japan and comparisons with other countries. Cancer Sci. 2007;98:1152–6.
Oki E, Zhao Y, Yoshida R, Egashira A, Ohgaki K, Morita M, et al. The difference in p53 mutations between cancers of the upper and lower gastrointestinal tract. Digestion. 2009;79:33–9.
Nguyen T, Brunson D, Crespi CL, Penman BW, Wishnok JS, Tannenbaum SR. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. 1992;89:3030–4.
Wink DA, Kasprzak KS, Maragos CM, Elespuru RK, Misra M, Dunams TM, et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science. 1991;254:1001–3.
Sugimura T, Tanaka N, Kawachi T, Kogure K, Fujimura S. Production of stomach cancer in dogs by N-methyl-N'-nitro-N-nitrosoguanidine. Gan. 1971;62:67.
Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–6.
Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nat Immunol. 2003;4:452–6.
Yoshikawa K, Okazaki IM, Eto T, Kinoshita K, Muramatsu M, Nagaoka H, et al. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–6.
Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58.
Bargonetti J, Friedman PN, Kern SE, Vogelstein B, Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991;65:1083–91.
Kern SE, Kinzler KW, Baker SJ, Nigro JM, Rotter V, Levine AJ, et al. Mutant p53 proteins bind DNA abnormally in vitro. Oncogene. 1991;6:131–6.
Kang YK, Morita S, Satoh T, Ryu MH, Chao Y, Kato K, et al. Exploration of predictors of benefit from nivolumab monotherapy for patients with pretreated advanced gastric and gastroesophageal junction cancer: post hoc subanalysis from the ATTRACTION-2 study. Gastric Cancer. 2022;25:207–17.
Acknowledgements
The authors thank all of the patients and their families who participated in this study; all SCRUM-Japan GI-SCREEN and GOZILA investigators and site personnel; Translational Research Support Section, National Cancer Center Hospital East, Kashiwa, Japan for study management and data centre support. The authors also thank Editage for their contribution to English proofreading for this manuscript.
Funding
This work was supported by grants from JSPS KAKENHI (Grant number JP21K08735 to KA) and the Japan Agency for Medical Research and Development (no. 19ck0106445h0002 to YN).
Author information
Authors and Affiliations
Contributions
KA, YN and HK contributed to the planning and conducting of studies, recruiting patients, acquisition of data, and writing of the manuscript. MS contributed to the analysis and interpretation of the data. DK, HB, TN, TY, SY, YN, HH, TO, TE, YH, KK, YY, KM, KO, NI, HK, TK, TS, NO and AT contributed to the recruitment of patients and acquisition of data. KY, TY, TM and EO contributed to the planning, conducting of studies and interpretation of data. All authors agree to be accountable for all aspects of the work and will ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
KA, MS, KO and YM declare no conflict of interest. YN received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Merck Biopharma, and Guardant Health AMEA and research grants from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd., Guardant Health, Genomedia, Seagen, and Roche Diagnostics. H Kitao received research funding from Taiho Pharmaceutical Co. Ltd. DK received honoraria for lectures from Takeda, Chugai, Eli Lilly, MSD, Ono, Taiho, Bristol Myers Squibb, Daiichi-Sankyo, Pfizer, Eisai, Merckbiopharma and Sysmex, and research funding from Ono, MSD, Novartis, Servier, Janssen, IQVIA, Syneos health, Cimic and Cimicshiftzero. HB received research funding from Ono, and honoraria for lectures from Ono, Taiho and Eli Lilly. TN received honoraria for lectures from Taiho Pharmaceutical, Chugai, Ono Pharmaceutical, Bristol-Meyers Squibb and Lilly; and research funding from Taiho Pharmaceutical, Chugai, Daiichi Sankyo, MSD, Ono Pharmaceutical, Bristol-Meyers Squibb, Lilly and Sumitomo Dainippon Pharma. TY received honoraria for lectures from Ono Pharmaceutical, Taiho Pharmaceutical, Johnson and Johnson, and Bristol-Myers Squibb K.K. SY received honoraria for lectures from Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., MSD K.K., Ono Phermaceutical Co., Ltd., Merck Biopharma Co., Ltd., Daiichi Sankyo Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., and Bristol-Myers Squibb K.K. YN received research funding from Chugai, MSD, Amgen, ONO Pharmaceutical, Astellas, Sanofi, Taiho, Eisai, Daiichi Sankyo, Novartis, Pfizer; Honoraria for lectures, presentations, and speakers’ bureaus from Yakult Honsha, Taiho, Eli Lilly, Daiichi Sankyo, Ono Pharma, Bristol-Mayers Squibb; Participation on an Advisory Board of Daiichi Sankyo. HH received honoraria from Bayer, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly, Merck Biopharma, MSD, Ono, Taiho, Takeda and Yakult, consulting or advisory roles Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, MSD and Ono, and research grants from ALX oncology, Amgen, Astellas, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Janssen, Merck Biopharma, MSD, Ono and Taiho. TO received honoraria for lectures from Eli Lilly, Bristol-Myers Squibb K.K., Taiho Pharmaceutical, Ono Pharmaceutical, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd. and Merck & Co., Inc., and research grants from Takeda Pharmaceutical Co., Ltd. TE. received honoraria for lectures from Daiichi Sankyo, Taiho and Chugai; and research funding from Novartis, Ono Pharmaceutical, Daiichi Sankyo, MSD, Astellas, Amgen Astellas BioPharma, IQVIA, Chugai, Pfyzer, Quitiles, Asahikasei Pharma and Syneos Health. YH received honoraria for lectures from Ono Pharmaceutical. KK received consulting fees from Daiichi-Sankyo, Seagen and Servier, honoraria for lectures from Ono Pharmaceutical, Bristol Myers Squibb, MSD and Taiho, research funding from Ono Pharmaceutical, Bristol Myers Squibb, MSD, Shionogi, Chugai, AstraZeneca, Janssen, Bayer, Merck Bio, Oncolys Biopharma and Beigene. YY received honoraria for lectures from Taiho Pharmaceutical, Ono Pharmaceutical, Asahi Kasei, Sanofi, Nihonkayaku, Merck Serono and Yakult, Eisai. KM received research grant from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K, Astellas, BeiGene and Daiichi Sankyo Co., Ltd. NI received honoraria for lectures from Bristol-Myers Squibb K.K., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., and MSD K.K. HK received honoraria for lectures from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Merck Biopharma Co., Ltd., Takeda Pharmaceutical Co. Ltd., Yakult Pharmaceutical Industry, Teijin Pharma Ltd., Incyte Biosciences Japan., and Taiho Pharmaceutical Co. Ltd.; lecture fees from Glaxo Smith Kline K.K., and Otsuka Pharmaceutical Co., Ltd.; and research funding from Kobayashi Pharmaceutical. Co., Ltd., and Eisai Co. Ltd. TK received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Yakult Honsha Co., Ltd., and Taiho Pharmaceutical Co., Ltd., and research grants from Chugai Pharmaceutical Co., Ltd. TS received honoraria for lectures from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Bristol-Myers Squibb K.K., Taiho Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd., research grants from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Bristol-Myers Squibb K.K., Taiho Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., MSD K.K., Gilead Sciences, Inc., and Parexel International Corporation, scholarship grants from Taiho Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd., and endowed chair from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. NO received honoraria for lectures from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd. Bayer Yakuhin, Ltd. and Eisai Co., Ltd; and advisory board from GlaxoSmithKline CO., Ltd. AT received speakers’ bureaux with Taiho Pharmaceutical, Chugai, Eli Lilly Japan, Merck Serono, Sanofi and Bristol-Myers Squibb; and research funding from Taiho Pharmaceutical, Sanofi and Ono Pharmaceutical. KY received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd. TY received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck Biopharma Co., Ltd., Bayer Yakuhin, Ltd., MSD K.K., and Ono Pharmaceutical Co., Ltd., and research grants from MSD K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Amgen K.K., Eisai Co., Ltd., FALCO biosystems Ltd., Genomedia Inc., Molecular Health GmbH, Nippon Boehringer Ingelheim Co., Ltd., Pfizer Japan Inc., Roche Diagnostics K., Sysmex Corp., and Sysmex Corp. EO received research funding from Guardant Health, and honoraria for lectures from Ono, Takeda, Bayer, Chugai, Taiho, Eli Lilly and Bristol Myers Squibb.
Ethical approval and consent to participate
The ethical, medical, and scientific aspects of the study were reviewed and approved by the Institutional Review Board of each institution and registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000016344). This study was conducted in accordance with the Declaration of Helsinki, revised in 2000, and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ando, K., Nakamura, Y., Kitao, H. et al. Mutational spectrum of TP53 gene correlates with nivolumab treatment efficacy in advanced gastric cancer (TP53MUT study). Br J Cancer 129, 1032–1039 (2023). https://doi.org/10.1038/s41416-023-02378-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02378-9