Abstract
Background
Therapeutic modalities including chemo, radiation, immunotherapy, etc. induce PD-L1 expression that facilitates the adaptive immune resistance to evade the antitumour immune response. IFN-γ and hypoxia are some of the crucial inducers of PD-L1 expression in tumour and systemic microenvironment which regulate the expression of PD-L1 via various factors including HIF-1α and MAPK signalling. Hence, inhibition of these factors is crucial to regulate the induced PD-L1 expression and to achieve a durable therapeutic outcome by averting the immunosuppression.
Methods
B16-F10 melanoma, 4T1 breast carcinoma, and GL261 glioblastoma murine models were established to investigate the in vivo antitumour efficacy of Ponatinib. Western blot, immunohistochemistry, and ELISA were performed to determine the effect of Ponatinib on the immunomodulation of tumour microenvironment (TME). CTL assay and flow cytometry were such as p-MAPK, p-JNK, p-Erk, and cleaved caspase-3 carried out to evaluate the systemic immunity induced by Ponatinib. RNA sequencing, immunofluorescence and Western blot analysis were used to determine the mechanism of PD-L1 regulation by Ponatinib. Antitumour immunity induced by Ponatinib were compared with Dasatinib.
Results
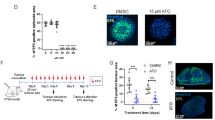
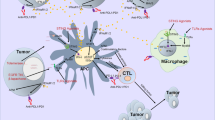
Here, Ponatinib treatment delayed the growth of tumours by inhibiting PD-L1 and modulating TME. It also downregulated the level of PD-L1 downstream signalling molecules. Ponatinib enhanced the CD8 T cell infiltration, regulated Th1/Th2 ratio and depleted tumour associated macrophages (TAMs) in TME. It induced a favourable systemic antitumour immunity by enhancing CD8 T cell population, tumour specific CTL activity, balancing the Th1/Th2 ratio and lowering PD-L1 expression. Ponatinib inhibited FoxP3 expression in tumour and spleen. RNA sequencing data revealed that Ponatinib treatment downregulated the genes related to transcription including HIF-1α. Further mechanistic studies showed that it inhibited the IFN-γ and hypoxia induced PD-L1 expression via regulating HIF-1α. Dasatinib was used as control to prove that Ponatinib induced antitumour immunity is via PD-L1 inhibition mediated T cell activation.
Conclusions
RNA sequencing data along with rigorous in vitro and in vivo studies revealed a novel molecular mechanism by which Ponatinib can inhibit the induced PD-L1 levels via regulating HIF-1α expression which leads to modulation of tumour microenvironment. Thus, our study provides a novel therapeutic insight of Ponatinib for the treatment of solid tumours where it can be used alone or in combination with other drugs which are known to induce PD-L1 expression and generate adaptive resistance.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All the materials used to produce the data in this study are available from the corresponding authors upon reasonable request.
References
Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21:3969–76.
Chen J, Jiang C, Jin L, Zhang X. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409–16.
Jalali S, Price-Troska T, Bothun C, Villasboas J, Kim H-J, Yang Z-Z, et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019;9:1–9.
Gato-Cañas M, Zuazo M, Arasanz H, Ibañez-Vea M, Lorenzo L, Fernandez-Hinojal G, et al. PDL1 signals through conserved sequence motifs to overcome interferon-mediated cytotoxicity. Cell Rep. 2017;20:1818–29.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64.
Gupta HB, Clark CA, Yuan B, Sareddy G, Pandeswara S, Padron AS, et al. Tumor cell-intrinsic PD-L1 promotes tumor-initiating cell generation and functions in melanoma and ovarian cancer. Signal Transduct Target Ther. 2016;1:1–9.
Escors D, Gato-Cañas M, Zuazo M, Arasanz H, García-Granda MJ, Vera R, et al. The intracellular signalosome of PD-L1 in cancer cells. Signal Transduct Target Ther. 2018;3:1–9.
Zerdes I, Matikas A, Bergh J, Rassidakis GZ, Foukakis T. Genetic, transcriptional and post-translational regulation of the programmed death protein ligand 1 in cancer: biology and clinical correlations. Oncogene. 2018;37:4639–61.
Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–74.
Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–68.
Zippelius A, Schreiner J, Herzig P, Müller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015;3:236–44.
Garcia-Diaz A, Shin DS, Moreno BH, Saco J, Escuin-Ordinas H, Rodriguez GA, et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017;19:1189–201.
Tan FH, Putoczki TL, Stylli SS, Luwor RB. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies. Onco Targets Ther. 2019;12:635.
O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–12.
Barnwal A, Das S, Bhattacharyya J. Repurposing ponatinib as a PD-L1 inhibitor revealed by drug repurposing screening and validation by in vitro and in vivo experiments. ACS Pharmacol Transl Sci. 2023;6:281–9. https://doi.org/10.1021/acsptsci.2c00214.
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116.
Cassetta L, Kitamura T. Targeting tumor-associated macrophages as a potential strategy to enhance the response to immune checkpoint inhibitors. Front Cell Dev Biol. 2018;6:38.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21.
Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10:823–44.
Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front Immunol. 2018;9:14.
Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:1–13.
Concha-Benavente F, Srivastava RM, Trivedi S, Lei Y, Chandran U, Seethala RR, et al. Identification of the cell-intrinsic and-extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 2016;76:1031–43.
Passariello M, D’Alise AM, Esposito A, Vetrei C, Froechlich G, Scarselli E, et al. Novel human anti-PD-L1 mAbs inhibit immune-independent tumor cell growth and PD-L1 associated intracellular signalling. Sci Rep. 2019;9:1–13.
Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta Mol Cell Res. 2007;1773:1358–75.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50.
Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:1–13.
Kiyomi A, Makita M, Ozeki T, Li N, Satomura A, Tanaka S, et al. Characterization and clinical implication of Th1/Th2/Th17 cytokines produced from three-dimensionally cultured tumor tissues resected from breast cancer patients. Transl Oncol. 2015;8:318–26.
Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462.
Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19:1–23.
Roux C, Jafari SM, Shinde R, Duncan G, Cescon DW, Silvester J, et al. Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc Natl Acad Sci USA 2019;116:4326–35.
Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59.
Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–20.
Lin Y-C, Chang L-Y, Huang C-T, Peng H-M, Dutta A, Chen T-C, et al. Effector/memory but not naive regulatory T cells are responsible for the loss of concomitant tumor immunity. J Immunol. 2009;182:6095–104.
Shigemori T, Toiyama Y, Okugawa Y, Yamamoto A, Yin C, Narumi A, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26:876–83.
Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–90.
Weichsel R, Dix C, Wooldridge L, Clement M, Fenton-May A, Sewell AK, et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res. 2008;14:2484–91. https://doi.org/10.1158/1078-0432.ccr-07-4393.
Flietner E, Wen Z, Rajagopalan A, Jung O, Watkins L, Wiesner J, et al. Ponatinib sensitizes myeloma cells to MEK inhibition in the high-risk VQ model. Sci Rep. 2022;12:10616. https://doi.org/10.1038/s41598-022-14114-z.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. https://doi.org/10.1038/nm730.
Granier C, Dariane C, Combe P, Verkarre V, Urien S, Badoual C, et al. Tim-3 expression on tumor-infiltrating PD-1(+)CD8(+) T cells correlates with poor clinical outcome in renal cell carcinoma. Cancer Res. 2017;77:1075–82. https://doi.org/10.1158/0008-5472.can-16-0274.
Roussel M, Le KS, Granier C, Llamas Gutierrez F, Foucher E, Le Gallou S, et al. Functional characterization of PD1+TIM3+ tumor-infiltrating T cells in DLBCL and effects of PD1 or TIM3 blockade. Blood Adv. 2021;5:1816–29. https://doi.org/10.1182/bloodadvances.2020003080.
Sawada M, Goto K, Morimoto-Okazawa A, Haruna M, Yamamoto K, Yamamoto Y, et al. PD-1+ Tim3+ tumor-infiltrating CD8 T cells sustain the potential for IFN-γ production, but lose cytotoxic activity in ovarian cancer. Int Immunol. 2020;32:397–405. https://doi.org/10.1093/intimm/dxaa010.
Pu Y, Ji Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front Immunol. 2022;13:874589. https://doi.org/10.3389/fimmu.2022.874589.
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007;104:3360–5.
Hsu M-C, Hsiao J-R, Chang K-C, Wu Y-H, Su I-J, Jin Y-T, et al. Increase of programmed death-1-expressing intratumoral CD8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Mod Pathol. 2010;23:1393–403.
Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–74.
Massi D, Brusa D, Merelli B, Ciano M, Audrito V, Serra S, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014;25:2433–42.
Massi D, Brusa D, Merelli B, Falcone C, Xue G, Carobbio A, et al. The status of PD-L1 and tumor-infiltrating immune cells predict resistance and poor prognosis in BRAFi-treated melanoma patients harboring mutant BRAFV600. Ann Oncol. 2015;26:1980–7.
Chen S, Crabill GA, Pritchard TS, McMiller TL, Wei P, Pardoll DM, et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J Immunther Cancer. 2019;7:1–12.
Gao Y, Yang J, Cai Y, Fu S, Zhang N, Fu X, et al. IFN‐γ‐mediated inhibition of lung cancer correlates with PD‐L1 expression and is regulated by PI3K‐AKT signaling. Int J Cancer. 2018;143:931–43.
Jiang X, Zhou J, Giobbie-Hurder A, Wargo J, Hodi FS. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res. 2013;19:598–609.
Narsale A, Moya R, Davies JD. Human CD4+ CD25+ CD127hi cells and the Th1/Th2 phenotype. Clin Immunol. 2018;188:103–12.
Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46:S52–S61.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22.
Bocanegra A, Blanco E, Fernandez-Hinojal G, Arasanz H, Chocarro L, Zuazo M, et al. PD-L1 in systemic immunity: unraveling its contribution to PD-1/PD-L1 blockade immunotherapy. Int J Mol Sci. 2020;21:5918.
Zhou L, Cha G, Chen L, Yang C, Xu D, Ge M. HIF1α/PD-L1 axis mediates hypoxia-induced cell apoptosis and tumor progression in follicular thyroid carcinoma. Onco Targets Ther. 2019;12:6461.
Van Duijn A, Willemsen KJ, Van Uden NO, Hoyng L, Erades S, Koster J, et al. A secondary role for hypoxia and HIF1 in the regulation of (IFNγ-induced) PD-L1 expression in melanoma. Cancer Immunol Immunother. 2022;71:529–40.
Minn AJ, Wherry EJ. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell. 2016;165:272–5.
Acknowledgements
We would like to acknowledge the animal facility at National Institute of Immunology, Delhi for allowing to perform the animal studies. AB acknowledges IIT Delhi for providing her doctoral fellowship. We also acknowledge Mr. Amit Kumar from ILBS for helping in IHC slide preparation.
Funding
This research was supported through a project by Department of Biotechnology sanctioned to JB (Grant No. BT/PR29866/NNT/28/1586/2018) and a faculty interdisciplinary research project by Indian Institute of Technology Delhi (Grant No. MI02200G) (to JB).
Author information
Authors and Affiliations
Contributions
AB and JB conceived and designed the experiments. AB performed the experiments. AB and JB analysed the data and wrote the paper. SD and RT helped with the animal studies. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All the animal experiments in this study has the ethical approval from the National Institute of Immunology institutional animal ethics committee.
Consent for publication
All authors have agreed to publish this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barnwal, A., Tamang, R., Sanjeev Das et al. Ponatinib delays the growth of solid tumours by remodelling immunosuppressive tumour microenvironment through the inhibition of induced PD-L1 expression. Br J Cancer 129, 1007–1021 (2023). https://doi.org/10.1038/s41416-023-02316-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02316-9