Abstract
Background
To date, single-agent immune checkpoint inhibitor (CPI) therapy has proven to be ineffective against biomarker-unselected extrapulmonary poorly differentiated neuroendocrine carcinomas (EP-PDNECs). The efficacy of CPI in combination with chemotherapy remains under investigation.
Methods
Patients with advanced, progressive EP-PDNECs were enrolled in a two-part study of pembrolizumab-based therapy. In Part A, patients received pembrolizumab alone. In Part B, patients received pembrolizumab plus chemotherapy. Primary endpoint: objective response rate (ORR). Secondary endpoints: safety, progression-free survival (PFS) and overall survival (OS). Tumours were profiled for programmed death-ligand 1 expression, microsatellite-high/mismatch repair deficient status, mutational burden (TMB), genomic correlates. Tumour growth rate was evaluated.
Results
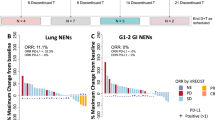
Part A (N = 14): ORR (pembrolizumab alone) 7% (95% CI, 0.2–33.9%), median PFS 1.8 months (95% CI, 1.7–21.4), median OS 7.8 months (95% CI, 3.1–not reached); 14% of patients (N = 2) had grade 3/4 treatment-related adverse events (TRAEs). Part B (N = 22): ORR (pembrolizumab plus chemotherapy) 5% (95% CI, 0–22.8%), median PFS 2.0 months (95% CI, 1.9–3.4), median OS 4.8 months (95% CI, 4.1–8.2); 45% of patients (N = 10) had grade 3/4 TRAEs. The two patients with objective response had high-TMB tumours.
Discussion
Treatment with pembrolizumab alone and pembrolizumab plus chemotherapy was ineffective in advanced, progressive EP-PDNECs.
Clinical trial registration
ClinicalTrials.gov NCT03136055.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data generated and analysed for this study are available within this manuscript, and the accompanying tables and figures, with the exception of identifiable information. Any additional inquiries should be referred to the corresponding author.
References
Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC. Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: a SEER database analysis of 162,983 cases. Cancer. 2018;124:807–15.
Shia J, Tang LH, Weiser MR, Brenner B, Adsay NV, Stelow EB, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am J Surg Pathol. 2008;32:719–31.
Volante M, Birocco N, Gatti G, Duregon E, Lorizzo K, Fazio N, et al. Extrapulmonary neuroendocrine small and large cell carcinomas: a review of controversial diagnostic and therapeutic issues. Hum Pathol. 2014;45:665–73.
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12.
Smith J, Reidy-Lagunes D. The management of extrapulmonary poorly differentiated (high-grade) neuroendocrine carcinomas. Semin Oncol. 2013;40:100–8.
Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–47.
Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, et al. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39:799–800.
McGarrah PW, Leventakos K, Hobday TJ, Molina JR, Finnes HD, Westin GF, et al. Efficacy of second-line chemotherapy in extrapulmonary neuroendocrine carcinoma. Pancreas. 2020;49:529–33.
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39.
Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38:2369–79.
Antonia SJ, Lopez-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95.
Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the CheckMate 032 randomized cohort. J Thorac Oncol. 2020;15:426–35.
Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens JHM, Kao S, Miller WH, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol. 2020;15:618–27.
Ott PA, Elez E, Hiret S, Kim DW, Morosky A, Saraf S, et al. Pembrolizumab in patients with extensive-stage small-cell lung cancer: results from the phase Ib KEYNOTE-028 study. J Clin Oncol. 2017;35:3823. -+
Vijayvergia N, Dasari A, Deng MY, Litwin S, Al-Toubah T, Alpaugh RK, et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122:1309–14.
Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer. 2021;28:161–72.
Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res. 2020;26:2290–6.
Capdevila J, Landolfi S, Hernando J, Teule A, Garcia-Carbonero R, Custodio A, et al. Durvalumab plus tremelimumab in patients with grade 3 neuroendocrine neoplasms of gastroenteropancreatic origin: updated results from the multicenter phase II DUNE trial (GETNE 1601). Ann Oncol. 2021;32:S914–S5.
Klein O, Kee D, Markman B, Michael M, Underhill C, Carlino MS, et al. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res. 2020;26:4454–9.
Girard N, Mazieres J, Otto J, Lena H, Lepage C, Egenod T, et al. Nivolumab (nivo) +/- ipilimumab (ipi) in pre-treated patients with advanced, refractory pulmonary or gastroenteropancreatic poorly differentiated neuroendocrine tumors (NECs) (GCO-001 NIPINEC). Ann Oncol. 2021;32:S1318. -S
Patel SP, Mayerson E, Chae YK, Strosberg J, Wang J, Konda B, et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART) SWOG S1609: high-grade neuroendocrine neoplasm cohort. Cancer Am Cancer Soc. 2021;127:3194–201.
Owen DH, Benner B, Wei L, Sukrithan V, Goyal A, Zhou Y, et al. Efficacy of nivolumab and temozolomide in advanced neuroendocrine neoplasms (NENs) in a phase 2 clinical trial. J Clin Oncol. 2022;40:4121.
Riesco-Martinez MC, Capdevila J, Alonso V, Jimenez-Fonseca P, Teule A, Grande E, et al. Nivolumab plus platinum-doublet chemotherapy as first-line therapy in unresectable, locally advanced or metastatic G3 neuroendocrine Neoplasms (NENs) of the gastroenteropancreatic (GEP) tract or unknown (UK) origin: Preliminary results from the phase II NICE-NEC trial (GETNE T1913). Ann Oncol. 2021;32:S908–S9.
Capdevila J, Teule A, Lopez C, Garcia-Carbonero R, Benavent M, Custodio A, et al. A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: the DUNE trial (GETNE 1601). Ann Oncol. 2020;31:S770–S1.
Fottner C, Apostolidis L, Ferrata M, Krug S, Michl P, Schad A, et al. A phase II, open label, multicenter trial of avelumab in patients with advanced, metastatic high-grade neuroendocrine carcinomas NEC G3 (WHO 2010) progressive after first-line chemotherapy (AVENEC). J Clin Oncol. 2019;37:4103.
Zhang P, Lu M, Li J, Shen L. Efficacy and safety of PD-1 blockade with JS001 in patients with advanced neuroendocrine neoplasms: a non-randomized, open-label, phase Ib trial. Ann Oncol. 2018;29:468.
Patel SP, Mayerson E, Chae YK, Strosberg J, Wang J, Konda B, et al. SWOG S1609-A phase 2 basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors: a high-grade neuroendocrine neoplasm cohort. Cancer. 2021;127:3194–201.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT) a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–64.
Gnirke A, Melnikov A, Maguire J, Rogov P, LeProust EM, Brockman W, et al. Solution hybrid selection with ultra-long oligonucleotides for massively parallel targeted sequencing. Nat Biotechnol. 2009;27:182–9.
Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol. 2011;29:3085–96.
Wagle N, Berger MF, Davis MJ, Blumenstiel B, DeFelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93.
Niu B, Ye K, Zhang Q, Lu C, Xie M, McLellan MD, et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics. 2014;30:1015–6.
Afshar AR, Damato BE, Stewart JM, Zablotska LB, Roy R, Olshen AB, et al. Next-generation sequencing of uveal melanoma for detection of genetic alterations predicting metastasis. Transl Vis Sci Technol. 2019;8:18.
Natesan D, Zhang L, Martell HJ, Jindal T, Devine P, Stohr B, et al. APOBEC mutational signature and tumor mutational burden as predictors of clinical outcomes and treatment response in patients with advanced urothelial cancer. Front Oncol. 2022;12:816706.
MacConaill LE, Garcia E, Shivdasani P, Ducar M, Adusumilli R, Breneiser M, et al. Prospective enterprise-level molecular genotyping of a cohort of cancer patients. J Mol Diagn. 2014;16:660–72.
Garcia EP, Minkovsky A, Jia Y, Ducar MD, Shivdasani P, Gong X, et al. Validation of OncoPanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–8.
Ferte C, Fernandez M, Hollebecque A, Koscielny S, Levy A, Massard C, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20:246–52.
Ferte C, Koscielny S, Albiges L, Rocher L, Soria JC, Iacovelli R, et al. Tumor growth rate provides useful information to evaluate sorafenib and everolimus treatment in metastatic renal cell carcinoma patients: an integrated analysis of the TARGET and RECORD phase 3 trial data. Eur Urol. 2014;65:713–20.
Dromain C, Pavel ME, Ruszniewski P, Langley A, Massien C, Baudin E, et al. Tumor growth rate as a metric of progression, response, and prognosis in pancreatic and intestinal neuroendocrine tumors. BMC Cancer. 2019;19:66.
Lamarca A, Crona J, Ronot M, Opalinska M, Lopez Lopez C, Pezzutti D, et al. Value of tumor growth rate (TGR) as an early biomarker predictor of patients’ outcome in neuroendocrine tumors (NET)—the GREPONET study. Oncologist. 2019;24:e1082–e90.
Lamarca A, Ronot M, Moalla S, Crona J, Opalinska M, Lopez CL, et al. Tumor growth rate as a validated early radiological biomarker able to reflect treatment-induced changes in neuroendocrine tumors: the GREPONET-2 study. Clin Cancer Res. 2019;25:6692–9.
Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–8.
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21:1353–65.
Andre T, Berton D, Curigliano G, Jimenez-Rodriguez B, Ellard S, Gravina A, et al. Efficacy and safety of dostarlimab in patients (pts) with mismatch repair deficient (dMMR) solid tumors: analysis of 2 cohorts in the GARNET study. J Clin Oncol. 2022;40:2587.
Su X, Zhou X, Xiao C, Peng W, Wang Q, Zheng Y. Complete response to immunotherapy combined with chemotherapy in a patient with gynecological mixed cancer mainly composed of small cell neuroendocrine carcinoma with high tumor mutational burden: a case report. Front Oncol. 2022;12:750970.
Kang NW, Tan KT, Li CF, Kuo YH. Complete and durable response to nivolumab in recurrent poorly differentiated pancreatic neuroendocrine carcinoma with high tumor mutational burden. Curr Oncol. 2021;28:4587–96.
Stimes N, Stanbery L, Albrethsen M, Trivedi C, Hamouda D, Dworkin L, et al. Small-cell breast carcinoma with response to atezolizumab: a case report. Immunotherapy. 2022;14:669–74.
Ricco B, Salati M, Reggiani Bonetti L, Dominici M, Luppi G. PD-1 blockade in deficient mismatch repair mixed adenoneuroendocrine carcinoma of the stomach: new hope for an orphan disease. Tumori. 2020;106:NP57–NP62.
Stueger A, Winder T, Tinguely M, Petrausch U, Helbling D. Metastatic mixed adenoneuroendocrine carcinoma of the colon with response to immunotherapy with pembrolizumab: a case report. J Immunother. 2019;42:274–7.
Whitman J, Kardosh A, Diaz L Jr, Fong L, Hope T, Onodera C, et al. Complete response and immune-mediated adverse effects with checkpoint blockade: treatment of mismatch repair-deficient colorectal neuroendocrine carcinoma. JCO Precis Oncol. 2019;3:1–7.
Puccini A, Poorman K, Salem ME, Soldato D, Seeber A, Goldberg RM, et al. Comprehensive genomic profiling of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs). Clin Cancer Res. 2020;26:5943–51.
Venizelos A, Elvebakken H, Perren A, Nikolaienko O, Deng W, Lothe IMB, et al. The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2021;29:1–14.
Raj N, Shah R, Stadler Z, Mukherjee S, Chou J, Untch B, et al. Real-time genomic characterization of metastatic pancreatic neuroendocrine tumors has prognostic implications and identifies potential germline actionability. JCO Precis Oncol. 2018;2:1–18.
Sun F, Grenert JP, Tan L, Van Ziffle J, Joseph NM, Mulvey CK, et al. Checkpoint inhibitor immunotherapy to treat temozolomide-associated hypermutation in advanced atypical carcinoid tumor of the lung. JCO Precis Oncol. 2022;6:e2200009.
Lee SM, Sung CO. Comprehensive analysis of mutational and clinicopathologic characteristics of poorly differentiated colorectal neuroendocrine carcinomas. Sci Rep. 2021;11:6203.
Yachida S, Totoki Y, Noe M, Nakatani Y, Horie M, Kawasaki K, et al. Comprehensive genomic profiling of neuroendocrine carcinomas of the gastrointestinal system. Cancer Discov. 2022;12:692–711.
Dromain C, Loaiza-Bonilla A, Mirakhur B, Beveridge TJR, Fojo AT. Novel tumor growth rate analysis in the randomized CLARINET study establishes the efficacy of lanreotide depot/autogel 120 mg with prolonged administration in indolent neuroendocrine tumors. Oncologist. 2021;26:e632–e8.
Weisbrod AB, Kitano M, Thomas F, Williams D, Gulati N, Gesuwan K, et al. Assessment of tumor growth in pancreatic neuroendocrine tumors in von Hippel Lindau syndrome. J Am Coll Surg. 2014;218:163–9.
Dromain C, Sundin A, Najran P, Vidal Trueba H, Dioguardi Burgio M, Crona J, et al. Tumor growth rate to predict the outcome of patients with neuroendocrine tumors: performance and sources of variability. Neuroendocrinology. 2021;111:831–9.
Pettersson OJ, Fross-Baron K, Crona J, Sundin A. Tumor growth rate in pancreatic neuroendocrine tumor patients undergoing PRRT with 177Lu-DOTATATE. Endocr Connect. 2021;10:422–31.
Ji Z, Peng Z, Gong J, Zhang X, Li J, Lu M, et al. Hyperprogression after immunotherapy in patients with malignant tumors of digestive system. BMC Cancer. 2019;19:705.
Ramon-Patino JL, Schmid S, Lau S, Seymour L, Gaudreau PO, Li JJN, et al. iRECIST and atypical patterns of response to immuno-oncology drugs. J Immunother Cancer. 2022;10:e004849.
Al-Toubah T, Halfdanarson T, Gile J, Morse B, Sommerer K, Strosberg J. Efficacy of ipilimumab and nivolumab in patients with high-grade neuroendocrine neoplasms. ESMO Open. 2022;7:100364.
Acknowledgements
We wish to thank all of the patients participating in this study and their caregivers, along with the investigators and research study teams at UCSF, MSK and DFCI. In addition, we would like to thank Merck for supporting this multicenter study through the Merck Investigator Studies Programme.
Funding
This work was supported and funded by Merck & Co.; additional support provided by the National Cancer Institute of the National Institutes of Health under Award Numbers P30CA082103 (USCF), P30CA008748 (MSK), and P30CA006516 (DFCI).
Author information
Authors and Affiliations
Contributions
NR, JAC and EKB conceived/designed the work that led to this submission, acquired data, and interpreted the results. SJW acquired data and interpreted results. RRA, SC, LF, JG, TAH, KPK, CKM, PNM, KP, DR-L and LZ interpreted the results. AM, SP and SvF acquired the data. All authors of this manuscript participated in drafting and/or revising the manuscript and approved the final manuscript version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Competing interests
Dr. Nitya Raj: research funding (ITM, Corcept Therapeutics), consulting/advisory boards (Ipsen Pharma, HRA Pharma, Progenics Pharmaceuticals, AAA). Dr. Jennifer Chan: consulting/advisory boards (TerSera, AAA, Curium), honorarium (Ipsen), stock ownership (Merck). Dr. Rahul Aggarwal: research funding (Merck). Dr. Lawrence Fong: research funding (Roche/Genentech, Abbvie, Bavarian Nordic, Bristol Myers Squibb, Dendreon, Janssen, Merck, Partner Therapeutics), advisory boards (Actym, Allector, Astra Zeneca, Atreca, Bioalta, Bolt, Bristol Myer Squibb, Daiichi Sankyo, Immunogenesis, Innovent, Merck, Merck KGA, Nutcracker, RAPT, Scribe, Senti, Soteria, Sutro, Roche/Genentech). Dr. Thomas Hope: research funding (Clovis Oncology, GE Healthcare, Philips, AAA), consulting (Bayer, Curium, ITM, RayzeBio), advisory boards (Blue Earth Diagnostics, Ipsen), stock ownership (RayzeBio). Dr. Claire Mulvey: research funding (Genentech). Dr. Pamela Munster: research funding (Merck). Dr. Kimberly Perez: consulting/scientific advisory boards (Lantheus, Helsinn/QED). Dr. Diane Reidy-Lagunes: research funding (Merck, Ipsen, Novartis). The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study was reviewed by the University of California San Francisco (UCSF), Memorial Sloan Kettering Cancer Center (MSK), and Dana-Farber Cancer Institute (DFCI) Institutional Review Boards and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent before study enrolment.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raj, N., Chan, J.A., Wang, S.J. et al. Pembrolizumab alone and pembrolizumab plus chemotherapy in previously treated, extrapulmonary poorly differentiated neuroendocrine carcinomas. Br J Cancer 129, 291–300 (2023). https://doi.org/10.1038/s41416-023-02298-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02298-8