Abstract
Background
Efficacy of endocrine therapy in HR+/HER2− metastatic breast cancer could differ depending on the presence of BRCA1/2 germline mutation.
Methods
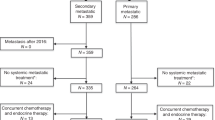
The ESME metastatic breast cancer platform (NCT03275311) is a French real world database. Multivariable models including a time-varying approach and landmark analyses assessed the association between time-dependent gBRCA status (categorised as gBRCAm, gBRCAwt (wild type), and untested), overall survival (OS), and first-line progression-free survival (PFS1).
Results
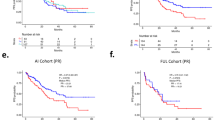
A total of 170 patients were gBRCAm carriers, 676 gBRCAwt, and 12,930 were untested at baseline. In the multivariable analysis, gBRCAm carriers overall had a lower OS compared to gBRCAwt (adjusted HR [95% CI] 1.26 [1.03–1.55]). gBRCAm patients treated with front-line endocrine therapy had lower adjusted OS (adjusted HR [95% CI] = 1.54 [1.03–2.32]) and PFS1 (adjusted HR [95% CI] 1.58 [1.17–2.12]) compared to gBRCAwt patients. However, for patients who received frontline chemotherapy, neither OS nor PFS1 differed between gBRCAm carriers and the other groups (HR versus gBRCAwt for OS: 1.12 [0.88–1.41], p = 0.350; PFS1: 1.09 [0.90–1.31], p = 0.379).
Conclusion
In this large cohort of HR+/HER2− MBC patients treated in a pre-CDK4/6 inhibitors era, gBRCAm status was associated with a lower OS and lower PFS following first-line endocrine therapy, but not following first-line chemotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data are contained in the ESME database, which is managed by Unicancer (http://www.unicancer.fr/). However, the ESME database is not publicly available for the following reason: in the ESME Research programme, public data sharing is not automatic in order to ensure that only trained users can analyse the ESME datasets. The analysis datasets will be made available only under data transfer and use agreements executed between Unicancer and the potential licensee. Interested parties should contact the corresponding author. Amélie Lusque had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data supporting the findings of this study are available from UNICANCER but restrictions apply to the availability of these data, which was used under licence for this study and is therefore not publicly available. However, the data are available from the authors upon reasonable request and with permission from UNICANCER.
Code availability
Codes used to generate the data are available upon reasonable request.
References
Mavaddat N, Barrowdale D, Andrulis IL, Domchek SM, Eccles D, Nevanlinna H, et al. Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the consortium of investigators of modifiers of BRCA1/2 (CIMBA). Cancer Epidemiol Biomark Prev. 2012;21:134–47.
Aleskandarany M, Caracappa D, Nolan CC, Macmillan RD, Ellis IO, Rakha EA, et al. DNA damage response markers are differentially expressed in BRCA-mutated breast cancers. Breast Cancer Res Treat. 2015;150:81–90.
Halpern N, Sonnenblick A, Uziely B, Divinsky L, Goldberg Y, Hamburger T, et al. Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int J Cancer. 2017;140:2145–9.
De Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A, et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020;10:7073.
Metcalfe K, Lynch HT, Foulkes WD, Tung N, Olopade OI, Eisen A, et al. Oestrogen receptor status and survival in women with BRCA2-associated breast cancer. Br J Cancer. 2019;120:398–403.
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl J Med. 2018;379:753–63.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl J Med. 2017;377:523–33.
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol: Off J Eur Soc Med Oncol / ESMO. 2020;31:1623–49.
Andre F, Su F, Solovieff N, Arteaga CL, Hortobagyi GN, Chia SKL, et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J Clin Oncol. 2020;38:1009.
Frenel JS, Dalenc F, Pistilli B, de La Motte Rouge T, Levy C, Mouret-Reynier MA, et al. 304P ESR1 mutations and outcomes in BRCA1/2 or PALB2 germline mutation carriers receiving first line aromatase inhibitor + palbociclib (AI+P) for metastatic breast cancer (MBC) in the PADA-1 trial. Ann Oncol. 2020;31:S364.
Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC, et al. A real-world evidence study of CDK4/6 inhibitor treatment patterns and outcomes in metastatic breast cancer by germline BRCA mutation status. Oncol Ther. 2021;9:575–89.
Bruno L, Ostinelli A, Waisberg F, Enrico D, Ponce C, Rivero S, et al. Cyclin-dependent kinase 4/6 inhibitor outcomes in patients with advanced breast cancer carrying germline pathogenic variants in DNA repair-related genes. JCO Precis Oncol. 2022;6:e2100140.
Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24.
Deluche E, Antoine A, Bachelot T, Lardy-Cleaud A, Dieras V, Brain E, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J cancer. 2020;129:60–70.
Quek RGW, Mardekian J. Clinical outcomes, treatment patterns, and health resource utilization among metastatic breast cancer patients with germline BRCA1/2 mutation: a real-world retrospective study. Adv Ther. 2019;36:708–20.
Baretta Z, Mocellin S, Goldin E, Olopade OI, Huo D. Effect of BRCA germline mutations on breast cancer prognosis: A systematic review and meta-analysis. Med (Baltim). 2016;95:e4975.
Rennert G, Bisland-Naggan S, Barnett-Griness O, Bar-Joseph N, Zhang S, Rennert HS, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–23.
Verhoog LC, Brekelmans CT, Seynaeve C, van den Bosch LM, Dahmen G, van Geel AN, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet 1998;351:316–21.
Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31:3191–6.
Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119:13–24.
van den Broek AJ, Schmidt MK, van ‘t Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: what’s the evidence? A systematic review with meta-analysis. PloS One. 2015;10:e0120189.
Schmidt MK, van den Broek AJ, Tollenaar RA, Smit VT, Westenend PJ, Brinkhuis M, et al. Breast cancer survival of BRCA1/BRCA2 mutation carriers in a hospital-based cohort of young women. J Natl Cancer Inst. 2017;109. https://doi.org/10.1093/jnci/djw329.
Copson ER, Maishman TC, Tapper WJ, Cutress RI, Greville-Heygate S, Altman DG, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169–80.
Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26:4282–8.
Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an international prospective breast cancer family registry population-based cohort study. J Clin Oncol: Off J Am Soc Clin Oncol. 2012;30:19–26.
Fasching PA, Yadav S, Hu C, Wunderle M, Haberle L, Hart SN, et al. Mutations in BRCA1/2 and other panel genes in patients with metastatic breast cancer -association with patient and disease characteristics and effect on prognosis. J Clin Oncol: Off J Am Soc Clin Oncol. 2021;39:1619–30.
Niyazov A, Quek RGW, Lewis K, Kemp J, Rider A. 161P - BRCA status, treatment patterns and outcomes in HER2- advanced breast cancer (ABC): A multi-country real-world study. Ann Oncol. 2019;30:iii51–iii2.
Safonov A, Bandlamudi C, Tallon de Lara P, Ferraro E, Derakhshan F, Will M et al. Comprehensive genomic profiling of patients with breast cancer identifies germline-somatic interactions mediating therapy resistance. Presented at SABCS 2021; December 7–10, 2021; San Antonio, TX. Abstract GS4-08.
Bertucci F, Ng CKY, Patsouris A, Droin N, Piscuoglio S, Carbuccia N, et al. Genomic characterization of metastatic breast cancers. Nature 2019;569:560–4.
Lips EH, Debipersad RD, Scheerman CE, Mulder L, Sonke GS, van der Kolk LE, et al. BRCA1-mutated estrogen receptor-positive breast cancer shows BRCAness, suggesting sensitivity to drugs targeting homologous recombination deficiency. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017;23:1236–41.
Marra A, Gazzo A, Gupta A, Selenica P, Da Silva EM, Pareja F, et al. 210O Mutational signature analysis reveals patterns of genomic instability linked to resistance to endocrine therapy (ET) +/− CDK 4/6 inhibition (CDK4/6i) in estrogen receptor-positive/HER2-negative (ER+/HER2-) metastatic breast cancer (MBC). Ann Oncol. 2022;33:S632.
Park YH, Im S-A, Park K, Wen J, Min A, Bonato V, et al. Prospective longitudinal multi-omics study of palbociclib resistance in hormone receptor+/HER2- metastatic breast cancer. J Clin Oncol. 2021;39:1013.
Acknowledgements
We thank the 18 French Comprehensive Cancer Centres for providing the data and each ESME local coordinator for managing the project at the local level. 18 Participating French Comprehensive Cancer Centres (FCCC):; I. Curie, Paris/ Saint-Cloud, G. Roussy, Villejuif, I. Cancérologie de l’Ouest, Angers/Nantes, C. F. Baclesse, Caen, ICM Montpellier, C. L. Bérard, Lyon, C. G-F Leclerc, Dijon, C. H. Becquerel, Rouen; I. C. Regaud, Toulouse; C. A. Lacassagne, Nice; Institut de Cancérologie de Lorraine, Nancy; C. E. Marquis, Rennes; I. Paoli-Calmettes, Marseille; C. J. Perrin, Clermont Ferrand; I. Bergonié, Bordeaux; C. P. Strauss, Strasbourg; I. J. Godinot, Reims; C. O. Lambret, Lille. Moreover, we thank the ESME Scientific Group, the Strategic Committee, and the ESME coordination team for their ongoing support. Prior presentations This work has been presented at the ESMO meeting as a poster in 2021.
Funding
The ESME MBC database receives financial support from an industrial consortium (Roche, Pfizer, AstraZeneca, MSD, Eisai, and Daiichi Sankyo). Data collection, analysis, and publication are managed entirely by UNICANCER independently of the industrial consortium.
Author information
Authors and Affiliations
Contributions
Conceptualisation JSF, AL, SD, AMa, WJ, TDLM. Formal analysis JSF, AL, SD, AMa, WJ, TDLM. Project administration MC. Investigation JSF, AL, SD, JMF, TB, ID, CL, JCE, AG, AP, MAM, JCT, TP, LC, LU, MD, MC, WJ, TDLM. Writing original draught JSF, AL, SD, AMa, WJ, TDLM. Writing review and editing JSF, AL, SD, JMF, TB, ID, CL, JCE, AG, AP, MAM, JCT, TP, LC, LU, MD, MC, WJ, TDLM
Corresponding author
Ethics declarations
Competing interests
JSF reports personal fees from Roche Genentech, personal fees and non-financial support from SeattleGenetics, personal fees and non-financial support from Novartis, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Lilly, personal fees and non-financial support from Novartis, personal fees and non-financial support from GSK, personal fees and non-financial support from Clovis oncoloy, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Daiichi Sankyo, personal fees and non-financial support from Gilead, personal fees and non-financial support from MSD, personal fees and non-financial support from Pierre Fabre, personal fees and non-financial support from Amgen, outside the submitted work. AL has declared no conflict of interest. SD reports grants and non-financial support from Pfizer, grants from Novartis, grants and non-financial support from Astra Zeneca, grants and non-financial support from Roche Genentech, grants from Lilly, grants from Puma, grants from Myriad, grants from Orion, grants from Amgen, grants from Sanofi, grants from Genomic Health, grants from GE, grants from Servier, grants from MSD, grants from BMS, grants from Pierre Fabre, grants from Seagen, grants from Exact Sciences, grants from Rappta, grants from Besins, grants from European Commission, grants from French government grants, grants from Fondation ARC, outside the submitted work. JMF reports personal fees from Pfizer, Novartis, Pierre Fabre, Lilly. TB reports personal fees and non-financial support from Roche, grants, personal fees and non-financial support from Astra Zeneca, grants, personal fees and non-financial support from Pfizer, grants and personal fees from SeaGen, grants, personal fees and non-financial support from Novartis, outside the submitted work; ID has declared no conflict of interest. CL has received reports personal fees from Daiichi, Astra Zeneca, Lilly, MSD. JCE has declared no conflict of interest. AG has declared no conflict of interest. AP reports from Lilly, other from Daiichi-Sankyo, other from Pfizer, other from Pierre Fabre, outside the submitted work; MAM has declared no conflict of interest. JCT has received reports personal fees from PFIZER, personal fees from MSD, personal fees from ASTRAZENECA, non-financial support from NOVARTIS, outside the submitted work. TP eports personal fees and non-financial support from Pfizer, personal fees from Lilly, personal fees and non-financial support from Novartis, personal fees and non-financial support from Astra-Zeneca, personal fees from Seagen, personal fees and non-financial support from Daiichi Sankyo, personal fees from Pierre Fabre, outside the submitted work. LC has declared no conflict of interest. LU has declared no conflict of interest. MD has declared no conflict of interest. MC has declared no conflict of interest. AM has declared no conflict of interest. WJ reports personal fees and non-financial support from Novartis, grants, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Roche Genentech, personal fees and non-financial support from Lilly, non-financial support from Sanofi, personal fees from Daiichi Sankyo, personal fees from MSD, personal fees from Rain Pharmaceuticals, non-financial support from Pierre Fabre, personal fees from Seagen, personal fees from BMS, personal fees and non-financial support from Eisai, personal fees and non-financial support from Pfizer, non-financial support from Glaxo Smithkline, non-financial support from Chugai Pharma, outside the submitted work. TDLM reports grants from Novartis, grants, personal fees and non-financial support from Astra Zeneca, personal fees and non-financial support from Roche Genentech, grants and personal fees from MSD, grants from Seagen, personal fees from Eisai, grants, personal fees and non-financial support from Pfizer, personal fees and non-financial support from Glaxo Smithkline, personal fees and non-financial support from CLOVIS ONCOLOGY, outside the submitted work.
Ethics approval and consent to participate
ESME MBC database was authorised by the French data protection authority (Registration ID 1704113 and authorisation N°DE-2013.-117).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frenel, JS., Lusque, A., Delaloge, S. et al. Efficacy of front-line treatment for hormone receptor-positive HER2-negative metastatic breast cancer with germline BRCA1/2 mutation. Br J Cancer 128, 2072–2080 (2023). https://doi.org/10.1038/s41416-023-02248-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02248-4