Abstract
Background
Trop-2 and Nectin-4 are transmembrane proteins overexpressed in many tumours and targets of antibody–drug conjugates (ADC). In metastatic colorectal cancer (mCRC), the role of Trop-2 and Nectin-4 has been poorly investigated.
Methods
Tumour samples of patients randomised in the phase III TRIBE2 were assessed for Trop-2 and Nectin-4 expression.
Results
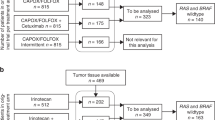
Three hundred eighty-six tumours were assessed for Trop-2 expression. 90 (23%), 115 (30%) and 181 (47%) were Trop-2 high, medium and low, respectively. Patients with low Trop-2 tumours achieved longer PFS (12 versus 9.9 months, p = 0.047) and OS (27.3 versus 21.3 months, p = 0.015) than those with high/medium Trop-2 tumours. These findings were confirmed in multivariate analysis (p = 0.022 and p = 0.023, respectively). A greater OS benefit from treatment intensification with FOLFOXIRI/bevacizumab was observed in patients with high/medium Trop-2 tumours (p-for-interaction = 0.041).
Two hundred fifty-one tumours were assessed for Nectin-4 expression. Fourteen (5%), 67 (27%) and 170 (68%) were high, medium and low, respectively. No prognostic impact was observed based on Nectin-4 expression and no interaction effect was reported between Nectin-4 expression groups and treatment arm.
Conclusions
In mCRC, expression levels of Trop-2 and Nectin-4 are heterogeneous, suggesting a target-driven development of anti-Trop2 and anti-Nectin-4 ADCs. Medium/high Trop-2 expression is associated with worse prognosis and higher benefit from chemotherapy intensification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Datasets supporting the results of this work are available to editors, referees and readers promptly upon request.
References
Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci USA 1981;78:5147–50.
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–72.
Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P, et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE. 2014;9:e96993.
Vidula N, Yau C, Rugo HS. Trop2 gene expression (Trop2e) in primary breast cancer (BC): correlations with clinical and tumor characteristics. JCO. 2017;35:1075–1075.
Avellini C, Licini C, Lazzarini R, Gesuita R, Guerra E, Tossetta G, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget. 2017;8:58642–53.
Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–91.
Pak MG, Shin DH, Lee CH, Lee MK. Significance of EpCAM and TROP2 expression in non-small cell lung cancer. World J Surg Oncol. 2012;10:53.
Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–5.
Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–63.
Mühlmann G, Spizzo G, Gostner J, Zitt M, Maier H, Moser P, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–8.
Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76:671–6.
Basu A, Goldenberg DM, Stein R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int J Cancer. 1995;62:472–9.
Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–33.
Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105.
Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9:28989–9006.
Zeng P, Chen MB, Zhou LN, Tang M, Liu CY, Lu PH. Impact of TROP2 expression on prognosis in solid tumors: a systematic review and meta-analysis. Sci Rep. 2016;6:33658.
Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–15.
Reymond N, Fabre S, Lecocq E, Adelaïde J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276:43205–15.
Hoffman-Censits JH, Lombardo KA, Parimi V, Kamanda S, Choi W, Hahn NM, et al. Expression of Nectin-4 in bladder urothelial carcinoma, in morphologic variants, and nonurothelial histotypes. Appl Immunohistochem Mol Morphol. 2021;29:619–25.
Lattanzio R, Ghasemi R, Brancati F, Sorda RL, Tinari N, Perracchio L, et al. Membranous Nectin-4 expression is a risk factor for distant relapse of T1-T2, N0 luminal-A early breast cancer. Oncogenesis. 2014;3:e118.
Zhang Y, Zhang J, Shen Q, Yin W, Huang H, Liu Y, et al. High expression of Nectin-4 is associated with unfavorable prognosis in gastric cancer. Oncol Lett. 2018;15:8789–95.
Zhang J, Liu K, Peng P, Li S, Ye Z, Su Y, et al. Upregulation of nectin‑4 is associated with ITGB1 and vasculogenic mimicry and may serve as a predictor of poor prognosis in colorectal cancer. Oncol Lett. 2019;18:1163–70.
Nishiwada S, Sho M, Yasuda S, Shimada K, Yamato I, Akahori T, et al. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J Exp Clin Cancer Res. 2015;34:30.
Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, Inai K, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009;69:6694–703.
Zhang Y, Liu S, Wang L, Wu Y, Hao J, Wang Z, et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett. 2016;375:179–89.
M-Rabet M, Cabaud O, Josselin E, Finetti P, Castellano R, Farina A, et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol. 2017;28:769–76.
Rajc J, Gugić D, Fröhlich I, Marjanović K, Dumenčić B. Prognostic role of Nectin-4 expression in luminal B (HER2 negative) breast cancer. Pathol Res Pract. 2017;213:1102–8.
Ma J, Sheng Z, Lv Y, Liu W, Yao Q, Pan T, et al. Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. Onco Targets Ther. 2016;9:183–90.
Starodub AN, Ocean AJ, Shah MA, Guarino MJ, Picozzi VJ, Vahdat LT, et al. First-in-human trial of a novel anti-trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res. 2015;21:3870–8.
Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Enfortumab vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–13.
Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–41.
Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee JL, et al. Enfortumab Vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–35.
FDA Grants Regular Approval to Sacituzumab Govitecan-hziy for Pretreated Patients With Triple-Negative Breast Cancer—The ASCO Post [Internet]. 2022. https://ascopost.com/issues/may-25-2021/fda-grants-regular-approval-to-sacituzumab-govitecan-hziy-for-pretreated-patients-with-triple-negative-breast-cancer/.
ESMO. EMA Recommends Granting a Marketing Authorisation for Sacituzumab Govitecan [Internet]. 2022. https://www.esmo.org/oncology-news/ema-recommends-granting-a-marketing-authorisation-for-sacituzumab-govitecan.
FDA. Research C for DE and FDA grants regular approval to enfortumab vedotin-ejfv for locally advanced or metastatic urothelial cancer. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-enfortumab-vedotin-ejfv-locally-advanced-or-metastatic-urothelial-cancer.
European Medicines Agency Accepts Marketing Authorization Application for Enfortumab Vedotin [Internet]. 2022. https://investor.seagen.com/press-releases/news-details/2021/European-Medicines-Agency-Accepts-Marketing-Authorization-Application-for-Enfortumab-Vedotin/default.aspx.
Peng LX, Zhao P, Zhao HS, Pan E, Yang BB, Li Q. Phosphoinositide 3-kinase/Akt pathway is involved in pingyangmycin‑induced growth inhibition, apoptosis and reduction of invasive potential in EOMA mouse hemangioendothelioma cells. Mol Med Rep. 2015;12:8275–81.
Cremolini C, Antoniotti C, Rossini D, Lonardi S, Loupakis F, Pietrantonio F, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507.
Bychkov A, Sampatanukul P, Shuangshoti S, Keelawat S. TROP-2 immunohistochemistry: a highly accurate method in the differential diagnosis of papillary thyroid carcinoma. Pathology. 2016;48:425–33.
Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, Yu EY, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. JCO. 2019;37:2592–600.
Birrer MJ, Moore KN, Betella I, Bates RC. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst. 2019;111:538–49.
Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao NS, et al. Antibody–drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res. 2019;25:5441–8.
Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody–drug conjugates: the last decade. Pharmaceuticals. 2020;13:245.
Peng J, Ou Q, Deng Y, Xiao B, Zhang L, Li J, et al. TROP2 overexpression in colorectal liver oligometastases is associated with poor prognosis after liver resection. Ther Adv Med Oncol. 2019;11:1758835919897543.
Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875–84.
Dum D, Taherpour N, Menz A, Höflmayer D, Völkel C, Hinsch A, et al. Trophoblast cell surface antigen 2 expression in human tumors: a tissue microarray study on 18,563 tumors. PAT. 2022;89:245–58.
Bardia A, Messersmith WA, Kio EA, Berlin JD, Vahdat L, Masters GA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol. 2021;32:746–56.
Gray JE, Heist RS, Starodub AN, Camidge DR, Kio EA, Masters GA, et al. Therapy of small cell lung cancer (SCLC) with a topoisomerase-i-inhibiting antibody-drug conjugate (ADC) targeting trop-2, Sacituzumab Govitecan. Clin Cancer Res. 2017;23:5711–9.
Siena S, Bartolomeo MD, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2021;22:779–89.
Heath EI, Rosenberg JE. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol. 2021;18:93–103.
Cheung CC, Banerjee D, Barnes PJ, Berendt RC, Butany J, Canil S, et al. Canadian Association of Pathologists–Association canadienne des pathologistes National Standards Committee for High Complexity Testing/Immunohistochemistry: Guidelines for the Preparation, Release, and Storage of Unstained Archived Diagnostic Tissue Sections for Immunohistochemistry. Am J Clin Pathol. 2014;142:629–33.
Grillo F, Pigozzi S, Ceriolo P, Calamaro P, Fiocca R, Mastracci L. Factors affecting immunoreactivity in long-term storage of formalin-fixed paraffin-embedded tissue sections. Histochem Cell Biol. 2015;144:93–9.
Das D, Satapathy SR, Siddharth S, Nayak A, Kundu CN. NECTIN-4 increased the 5-FU resistance in colon cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother Pharm. 2015;76:471–9.
Sethy C, Goutam K, Nayak D, Pradhan R, Molla S, Chatterjee S, et al. Clinical significance of a pvrl 4 encoded gene Nectin-4 in metastasis and angiogenesis for tumor relapse. J Cancer Res Clin Oncol. 2020;146:245–59.
Nicolò E, Giugliano F, Ascione L, Tarantino P, Corti C, Tolaney SM, et al. Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives. Cancer Treatment Reviews [Internet]. 2022. https://www.cancertreatmentreviews.com/article/S0305-7372(22)00059-7/fulltext.
Acknowledgements
We are grateful to GONO and ARCO Foundations, to all participating patients and their families, and to the GONO investigators from the participating Italian centres.
Funding
The study was supported by GONO and ARCO Foundations (no grant number applicable). The sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Study concepts: RM, CU, MMG, CC. Study design: RM, CU, MMG, MG, CC. Data acquisition: CU, MMG, MG. Quality control of the data and algorithms: CU, MMG, MG. Data analysis and interpretation: RM, CU, MMG, MG, CC. Statistical analysis: RM, MMG. Manuscript preparation: RM, MMG. Manuscript editing: CU, GF, CC. Manuscript review: all authors.
Corresponding author
Ethics declarations
Competing interests
FP: Honoraria—Amgen, Merck-Serono, Sanofi, Lilly, Bayer, Servier, Astrazeneca, MSD; Research Grants—Astrazeneca, BMS, Incyte. SL: Speakers’ Bureau—Amgen, Merck, Roche, Lilly, Bristol-Myers Squibb, Pierre-Fabre, GSK and Servier. Consulting or advisory role—Amgen, MSD, Merck Serono, Lilly, Astra Zeneca, Incyte, Daiichi-Sankyo, Bristol-Myers Squibb, Servier, Research Grants—Bayer, Merck, Amgen, Roche, Lilly, Astra Zeneca Bristol-Myers Squibb. FB: Honoraria—Lilly. Travel, accommodations and expenses—Bayer, Ipsen. GM: Honoraria—Amgen, Hoffmane-La Roche, Bayer, Merk Serono, Sirtex. CC: Honoraria—Amgen, Bayer, Merck, Roche and Servier. Consulting or advisory role—Amgen, Bayer, MSD, Roche. Speakers’ Bureau—Servier. Research funding—Bayer, Merck, Servier. Travel, accommodations and expenses—Roche and Servier. The remaining authors declared no competing interests.
Ethics approval and consent to participate
All patients provided written informed consent to study procedures before enrolment. Approvals for TRIBE protocol were obtained from local ethics committees of participating sites.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moretto, R., Germani, M.M., Giordano, M. et al. Trop-2 and Nectin-4 immunohistochemical expression in metastatic colorectal cancer: searching for the right population for drugs’ development. Br J Cancer 128, 1391–1399 (2023). https://doi.org/10.1038/s41416-023-02180-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02180-7