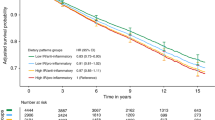
Abstract
Background
Chronic inflammation is implicated in cancer prognosis and can be modulated by diet. We examined associations between post-diagnosis dietary inflammatory potential and mortality outcomes among post-menopausal women diagnosed with cancer in the Women’s Health Initiative (WHI).
Methods
Energy-adjusted dietary inflammatory index scores (E-DII) were calculated from dietary and supplemental intake data collected on the first food frequency questionnaire following the diagnosis of primary invasive cancer for 3434 women in the WHI. Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for risk of death from any cause, cancer, cardiovascular disease (CVD) and other causes by post-diagnosis quartiles of E-DII. Subgroup analyses by cancer stage and grade were performed.
Results
There were 1156 deaths after a median 13 years of follow-up from the date of a cancer diagnosis. In the multivariable-adjusted analyses, a more anti-inflammatory diet plus supplements after cancer diagnosis was associated with lower all-cause mortality, cancer mortality, CVD mortality and mortality from other causes with HRsQ1vs.Q4 ranging from 0.47 to 0.68 (all P-trends < 0.05). Associations were stronger for cancers diagnosed at more distant stages or moderately differentiated grades.
Conclusion
A more anti-inflammatory diet plus supplements after a cancer diagnosis may improve survival for post-menopausal cancer survivors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
WHI data are available upon submission of a written proposal and approval by the WHI Publications and Presentations Committee.
References
Society AC. Cancer Treatment & Survivorship Facts & Figures 2019-2021. Atlanta: American Cancer Society; 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf.
Zheng J, Tabung FK, Zhang J, Liese AD, Shivappa N, Ockene JK, et al. Association between post-cancer diagnosis dietary inflammatory potential and mortality among invasive breast cancer survivors in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2018;27:454–63.
Blanchard CM, Denniston MM, Baker F, Ainsworth SR, Courneya KS, Hann DM, et al. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003;27:246–56.
Al Ramadhani RM, Nagle CM, Ibiebele TI, Grant P, Friedlander M, DeFazio A, et al. Pre-and post-diagnosis diet quality and ovarian cancer survival. Cancer Epidemiol Prev Biomarkers. 2021;30:229–32.
Guinter MA, McCullough ML, Gapstur SM, Campbell PT. Associations of pre-and postdiagnosis diet quality with risk of mortality among men and women with colorectal cancer. J Clin Oncol. 2018;36:3404.
George SM, Irwin ML, Smith AW, Neuhouser ML, Reedy J, McTiernan A, et al. Postdiagnosis diet quality, the combination of diet quality and recreational physical activity, and prognosis after early-stage breast cancer. Cancer Causes Control. 2011;22:589–98.
Tabung FK, Noonan A, Lee DH, Song M, Clinton SK, Spakowicz D, et al. Post-diagnosis dietary insulinemic potential and survival outcomes among colorectal cancer patients. BMC Cancer. 2020;20:1–12.
Leeming RC, Karagas MR, Gilbert-Diamond D, Emond JA, Zens MS, Schned AR, et al. Diet quality and survival in a population-based bladder cancer study. Nutr Cancer. 2022;74:2400–11.
Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74:737–48.
McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717–27.
Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96.
Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–96.
Byrd DA, Judd SE, Flanders WD, Hartman TJ, Fedirko V, Bostick RM. Development and validation of novel dietary and lifestyle inflammation scores. J Nutr. 2019;149:2206–18.
Kaluza J, Harris H, Melhus H, Michaelsson K, Wolk A. Questionnaire-based anti-inflammatory diet index as a predictor of low-grade systemic inflammation. Antioxid Redox Signal. 2018;28:78–84.
Tabung FK, Smith-Warner SA, Chavarro JE, Fung TT, Hu FB, Willett WC, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr. 2017;147:1567–77.
Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25:398–405.
Zheng J, Tabung FK, Zhang J, Murphy EA, Shivappa N, Ockene JK, et al. Post-cancer diagnosis dietary inflammatory potential is associated with survival among women diagnosed with colorectal cancer in the Women’s Health Initiative. Eur J Nutr. 2020;59:965–77.
Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109.
Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77.
Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13:S122–128.
White E, Shattuck AL, Kristal AR, Urban N, Prentice RL, Henderson MM, et al. Maintenance of a low-fat diet: follow-up of the Women’s Health Trial. Cancer Epidemiol Biomarkers Prev. 1992;1:315–23.
Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87.
Schakel S, Sievert Y, Buzzard I. Sources of data for developing and maintaining a nutrient database. J Am Dietetic Assoc. 1988;88:1268–71.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S.
George SM, Ballard-Barbash R, Shikany JM, Caan BJ, Freudenheim JL, Kroenke CH, et al. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2014;23:575–83.
Seidell JC, Flegal KM. Assessing obesity: classification and epidemiology. Br Med Bull. 1997;53:238–52.
Fritz A, Ries L. SEER Program Code Manual. Bethesda: Cancer Statistics Branch, Surveillance Program, Div of Cancer Control and Pop Sciences, National Cancer Institute, National Institutes of Health. Public Health Service, US Dept of Health and Human Services 3, 1998.
Bernardo BM, Pennell ML, Naughton MJ, Brodin NP, Neuhouser ML, Chlebowski RT, et al. Self-reported symptoms among cancer survivors in the Women’s Health Initiative (WHI) Life and Longevity after Cancer (LILAC) cohort. J Cancer Surviv. 2022. https://doi.org/10.1007/s11764-022-01200-4.
Paskett ED, Caan BJ, Johnson L, Bernardo BM, Young GS, Pennell ML, et al. The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) Study: description and baseline characteristics of participants. Cancer Epidemiol Biomarkers Prev. 2018;27:125–37.
Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–59.
Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67:145–53.
Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57.
Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–64.
Bo S, Ciccone G, Castiglione A, Gambino R, De Michieli F, Villois P, et al. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr Med Chem. 2013;20:1323–31.
Sears B. Anti-inflammatory diets for obesity and diabetes. J Am Coll Nutr. 2009;28:482s–491s.
Jang H, Chung MS, Kang SS, Park Y. Association between the dietary inflammatory index and risk for cancer recurrence and mortality among patients with breast cancer. Nutrients. 2018;10:1095.
Wang K, Sun J-Z, Wu Q-X, Li Z-Y, Li D-X, Xiong Y-F, et al. Long-term anti-inflammatory diet in relation to improved breast cancer prognosis: a prospective cohort study. npj Breast Cancer. 2020;6:1–11.
Ratjen I, Shivappa N, Schafmayer C, Burmeister G, Nöthlings U, Hampe J, et al. Association between the dietary inflammatory index and all-cause mortality in colorectal cancer long-term survivors. Int J Cancer. 2019;144:1292–301.
Castro-Espin C, Agudo A. The role of diet in prognosis among cancer survivors: a systematic review and meta-analysis of dietary patterns and diet interventions. Nutrients. 2022;14:348.
Zucchetto A, Gini A, Shivappa N, Hébert JR, Stocco C, Dal Maso L, et al. Dietary inflammatory index and prostate cancer survival. Int J Cancer. 2016;139:2398–404.
Nagle CM, Ibiebele T, Shivappa N, Hébert JR, DeFazio A, Webb PM, et al. The association between the inflammatory potential of diet and risk of developing, and survival following, a diagnosis of ovarian cancer. Eur J Nutr. 2019;58:1747–56.
Karavasiloglou N, Pestoni G, Faeh D, Rohrmann S. Post-diagnostic diet quality and mortality in females with self-reported history of breast or gynecological cancers: Results from the third national health and nutrition examination survey (NHANES III). Nutrients. 2019;11:2558.
Wang T, Farvid MS, Kang JH, Holmes MD, Rosner BA, Tamimi RM, et al. Diabetes risk reduction diet and survival after breast cancer diagnosis. Cancer Res. 2021;81:4155–62.
Yuan C, Bao Y, Sato K, Nimptsch K, Song M, Brand-Miller JC, et al. Influence of dietary insulin scores on survival in colorectal cancer patients. Br J Cancer. 2017;117:1079–87.
Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol Prev Biomarkers. 2013;22:792–802.
Pierce JP, Natarajan L, Caan BJ, Parker BA, Greenberg ER, Flatt SW, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:289–98.
Chlebowski RT, Blackburn GL, Hoy MK, Thomson CA, Giuliano AE, McAndrew P, et al. Survival analyses from the Women’s Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. J Clin Oncol. 2008;26:522–522.
Chlebowski RT, Aragaki AK, Anderson GL, Pan K, Neuhouser ML, Manson JE, et al. Dietary modification and breast cancer mortality: long-term follow-up of the Women’s Health Initiative randomized trial. J Clin Oncol. 2020;38:1419.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–14.
Solas M, Milagro FI, Ramírez MJ, Martínez JA. Inflammation and gut-brain axis link obesity to cognitive dysfunction: plausible pharmacological interventions. Curr Opin Pharmacol. 2017;37:87–92.
Argilés JM, Busquets S, Toledo M, López-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care. 2009;3:263–8.
Griffiths K, Aggarwal BB, Singh RB, Buttar HS, Wilson D, De Meester F. Food antioxidants and their anti-inflammatory properties: a potential role in cardiovascular diseases and cancer prevention. Diseases. 2016;4:28.
Shrihari TG. Dual role of inflammatory mediators in cancer. Ecancermedicalscience. 2017;11:721.
Sengupta S, Bhattacharyya D, Kasle G, Karmakar S, Sahu O, Ganguly A, et al. Potential immunomodulatory properties of biologically active components of spices against SARS-CoV-2 and Pan β-Coronaviruses. Front Cell Infect Microbiol. 2021;11:729622.
Hébert JR, Shivappa N, Wirth MD, Hussey JR, Hurley TG. Perspective: The Dietary Inflammatory Index (DII)—lessons learned, improvements made, and future directions. Adv Nutr. 2019;10:185–95.
Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Tylavsky FA, et al. Longitudinal changes in the dietary inflammatory index: an assessment of the inflammatory potential of diet over time in postmenopausal women. Eur J Clin Nutr. 2016;70:1374–80.
Tabung FK, Steck SE, Liese AD, Zhang J, Ma Y, Caan B, et al. Association between dietary inflammatory potential and breast cancer incidence and death: results from the Women’s Health Initiative. Br J Cancer. 2016;114:1277–85.
Acknowledgements
We thank the Women’s Health Initiative Investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota, Minneapolis, MN) Karen L. Margolis. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Mark Espeland. Additional Information: A full list of all the investigators who have contributed to Women’s Health Initiative science appears at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf. We also thank the Women’s Health Initiative staff and the trial participants for their outstanding dedication and commitment.
Funding
J Zheng, FKT, J Zhang, JKO, JRH and SES were supported by grant #318258 from the American Institute for Cancer Research. J Zheng was supported by National Natural Science Foundation of China 82103809 and Shanghai Pujiang Program 20PJ1409600. FKT was supported by National Cancer Institute grant # K99CA207736. NS and JRH were supported by grant #R44 DK103377 from the National Institute of Diabetes and Digestive and Kidney Diseases. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C and HHSN268201600004C.
Author information
Authors and Affiliations
Contributions
Conception and design: J Zheng, FKT, JRH, SES. Development of methodology: J Zheng, J Zhang, JRH, NS, SES. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): BC, CHK. Analysis and interpretation of data (e.g. statistical analysis, biostatistics, computational analysis): J Zheng, J Zhang, SES. Writing, review and/or revision of the manuscript: J Zheng, FKT, J Zhang, BC, JRH, CHK, JKO, NS, SES. Study supervision: SES.
Corresponding author
Ethics declarations
Competing interests
JRH owns controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the dietary inflammatory index (DIITM) from the University of South Carolina to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. NS is an employee of CHI. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The WHI protocol was approved by the Institutional Review Boards at the Clinical Coordinating Center (CCC) at the Fred Hutchinson Cancer Research Center (Seattle, WA) and at each of the participating Clinical Centers and conformed to the Declaration of Helsinki. All participants provided written informed consent in accordance with the U.S. Common Rule.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, J., Tabung, F.K., Zhang, J. et al. Association between dietary inflammatory potential and mortality after cancer diagnosis in the Women’s Health Initiative. Br J Cancer 128, 606–617 (2023). https://doi.org/10.1038/s41416-022-02079-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02079-9
This article is cited by
-
Integrative Oncology Approaches to Supporting Immune Checkpoint Inhibitor Treatment of Solid Tumours
Current Oncology Reports (2024)