Abstract
Background
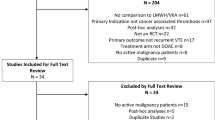
Clinical guidelines indicate that in patients with cancer-associated thrombosis (CAT), anticoagulant treatment should be continued beyond 6 months as long as the cancer is active. We aimed to analyse the safety of low-molecular-weight heparin (LMWH) beyond 12 months in patients with CAT.
Methods
We performed a post hoc analysis of consecutive CAT patients from October 2008 to December 2019. The primary outcome was the rate of clinically relevant bleeding (CRB), and we compared two periods (1–12 vs. 12–24 months). Hazard ratio (HR), competing risk analysis and sensitivity analyses were performed.
Results
Of the 588 patients included, 30.1% (n = 177) received LMWH beyond 12 months. The rate of CRB in the first 12 months compared to the 12–24 month period was 3.2 per 100 patients/month (95% CI 2.5–4.1) vs. 0.9 per 100 patients/month (95% CI 0.4–1.5), (P < 0.0001). The competing risk analysis of CRB comparing both periods showed a lower sub-distribution hazard ratio (SHR) during the period 12–24 months (SHR: 0.5, 95% CI: 0.3–0.8, P < 0.001).
Conclusion
In patients with cancer-associated thrombosis under anticoagulant treatment with LMWH, the rate of clinically relevant bleeding and major bleeding were lower beyond 12 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
References
Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thrombosis Haemostasis. 2017; 117:219–30.
Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer-A cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–13.
Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23.
Gussoni G, Frasson S, la Regina M, di Micco P, Monreal M. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thrombosis Res. 2013;131:24–30.
Ay C, Beyer-Westendorf J, Pabinger I. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019;30:897–907.
Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566–81.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52.
Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2019;38:496–52.
Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, Asensio-Cruz M, Blasco-Esquivias I, et al. Tinzaparin in cancer associated thrombosis beyond 6 months: TiCAT study. Thrombosis Res. 2017;157:90–6.
Francis CW, Kessler CM, Goldhaber SZ, Kovacs MJ, Monreal M, Huisman MV, et al. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN study. J Thrombosis Haemost. 2015;13:1028–35.
di Nisio M, van Es N, Carrier M, Wang TF, Garcia D, Segers A, et al. Extended treatment with edoxaban in cancer patients with venous thromboembolism: a post-hoc analysis of the Hokusai-VTE Cancer study. J Thromb Haemost. 2019;17:1866–74.
Marshall A, Levine M, Hill C, Hale D, Thirlwall J, Wilkie V, et al. Treatment of cancer-associated venous thromboembolism: 12-month outcomes of the placebo versus rivaroxaban randomization of the SELECT-D trial (SELECT-D: 12m). J Thromb Haemost. 2020;18:905–15.
Jara-Palomares L, Solier-Lopez A, Elias-Hernandez T, Asensio-Cruz MI, Blasco-Esquivias I, Sanchez-Lopez V, et al. D-dimer and high-sensitivity C-reactive protein levels to predict venous thromboembolism recurrence after discontinuation of anticoagulation for cancer-associated thrombosis. Br J Cancer. 2018;119:915–21.
Marin-Barrera L, Muñoz-Martin AJ, Rios-Herranz E, Garcia-Escobar I, Beato C, Font C, et al. A case-control analysis of the impact of venous thromboembolic disease on quality of life of patients with cancer: quality of life in cancer (QCA) study. Cancers. 2019;12:75.
Lyman GH, Khorana AA, Falanga A, Clarke-Pearson D, Flowers C, Jahanzeb M, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505.
Konstantinides SV, Meyer G, Bueno H, Galié N, Gibbs JSR, Ageno W, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. 2020;41:543–603.
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–80.
Mazzolai L, Aboyans V, Ageno W, Agnelli G, Alatri A, Bauersachs R, et al. Diagnosis and management of acute deep vein thrombosis: a joint consensus document from the European Society of Cardiology working groups of aorta and peripheral vascular diseases and pulmonary circulation and right ventricular function. Eur Heart J. 2018;39:4208–18.
Mazzolai L, Ageno W, Alatri A, Bauersachs R, Becattini C, Brodmann M, et al. Second consensus document on diagnosis and management of acute deep vein thrombosis: updated document elaborated by the ESC Working Group on aorta and peripheral vascular diseases and the ESC Working Group on pulmonary circulation and right ventricular function. Eur J Prev Cardiol. 2022;29:1248–63.
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J Thrombosis Haemost. 2015;13:2119–26.
Lee AYY, Levine MN, Baker RI, Bowden C, Kakkar AK, Prins M, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl J Med. 2003;349:146–53.
Caiano L, Carrier M, Marshall A, Young AM, Ageno W, Delluc A, et al. Outcomes among patients with cancer and incidental or symptomatic venous thromboembolism: A systematic review and meta-analysis. J Thrombosis Haemostasis. 2021;19:2468–79.
Kraaijpoel N, Bleker SM, Meyer G, Mahé I, Muñoz A, Bertoletti L, et al. Treatment and long-term clinical outcomes of incidental pulmonary embolism in patients with cancer: an international prospective cohort study. J Clin Oncol. 2019;37:1713–20.
Acknowledgements
We would like to thank Henry Antonio Andrade Ruiz, from the Methodological and Statistical Support Unit, for his support and contribution to the statistical analysis.
Funding
This project was supported by LEO Pharma Research Foundation.
Author information
Authors and Affiliations
Contributions
Conception and design: MB-H, SL-R, SM-R and LJ-P. Data analysis and interpretation: MB-H, SL-R, SM-R and LJ-P. Synthesis of the results: all authors. Manuscript writing: SL-R and LJ-P. Manuscripts review and edits. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Corresponding author
Ethics declarations
Competing interests
LJ-P reports personal fees from Bayer Hispania, Actelion, Rovi, Pfizer, Menarini, and Leo Pharma, outside the submitted work. ROC reports grant support from Leo Pharma and Bayer Healthcare, and fees for serving on advisory boards and giving lectures from Leo Pharma, Rovi, Bayer Healthcare, MSD and Actelion. There are no other competing interests.
Ethics approval and consent to participate
This post hoc analysis was evaluated and approved by the Ethical Committee of the centre according to Spanish Regulatory Authorities (0511-N-22). This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation (ICH) Guidelines for Good Clinical Practice and in full conformity with relevant regulations. Documents constituting the master file of the study included all the documents established in good clinical practice (CPMP/ICH/135/95). In this project, the collection, process and analysis of all data were anonymously carried out, and only for the purposes of the project. All data were protected in accordance with the European Union directive 2016/679 of the European Parliament and the European Council, of April 27, 2016, regarding the protection of persons and their personal data.
Consent to publish
No publication consent is required for this specific study. This post hoc analysis was evaluated and approved by the Ethical Committee of the centre according to Spanish Regulatory Authorities (0511-N-22). Original prospective studies required informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lopez-Ruz, S., Barca-Hernando, M., Marin-Romero, S. et al. Low-molecular-weight heparin beyond 12 months in patients with cancer-associated thrombosis. Br J Cancer 127, 2234–2240 (2022). https://doi.org/10.1038/s41416-022-02007-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-02007-x