Abstract
Background
Lysine acetyltransferase 6 A (KAT6A) is a MYST-type histone acetyltransferase (HAT) enzyme, which contributes to histone modification and cancer development. However, its biological functions and molecular mechanisms, which respect to hepatocellular carcinoma (HCC), are still largely unknown.
Methods
Immunohistochemical, western blot and qRT-PCR analysis of KAT6A were performed. A series of in vitro and in vivo experiments were conducted to reveal the role of KAT6A in the progression of HCC.
Results
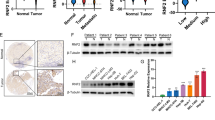
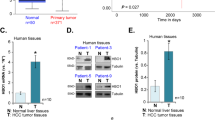
We demonstrated that KAT6A expression was upregulated in HCC tissues and cell lines. Clinical analysis showed that increased KAT6A was significantly associated with malignant prognostic features and shorter survival. Gain- and loss-of-function experiments indicated that KAT6A promoted cell viability, proliferation and colony formation of HCC cells in vitro and in vivo. We confirmed that KAT6A acetylates lysine 23 of histone H3 (H3K23), and then enhances the association of the nuclear receptor binding protein TRIM24 and H3K23ac. Consequently, TRIM24 functions as a transcriptional activator to activate SOX2 transcription and expression, leading to HCC tumorigenesis. Restoration of SOX2 at least partially abolished the biological effects of KAT6A on HCC cells. Overexpression of KAT6A acetyltransferase activity-deficient mutants or TRIM24 mutants lacking H3K23ac binding sites did not affect SOX2 expression and HCC biological function. Moreover, matrix stiffness can upregulate the expression of KAT6A in HCC cells.
Conclusions
Our data support the first evidence that KAT6A plays an oncogenic role in HCC through H3K23ac/TRIM24-SOX2 pathway, and represents a promising therapeutic strategy for HCC patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Nakamura M, Chiba T, Kanayama K, Kanzaki H, Saito T, Kusakabe Y, et al. Epigenetic dysregulation in hepatocellular carcinoma: an up-to-date review. Hepatol Res. 2019;49:3–13.
Borrow J, Stanton VP Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41.
Huang F, Abmayr SM, Workman JL. Regulation of KAT6 acetyltransferases and their roles in cell cycle progression, stem cell maintenance, and human disease. Mol Cell Biol. 2016;36:1900–7.
Lian J, Zhang H, Wei F, Li Q, Lu Y, Yu B, et al. Long non-coding RNA DANCR promotes colorectal tumor growth by binding to lysine acetyltransferase 6A. Cell Signal. 2020;67:109502.
Baell JB, Leaver DJ, Hermans SJ, Kelly GL, Brennan MS, Downer NL, et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature. 2018;560:253–7.
Yu L, Liang Y, Cao X, Wang X, Gao H, Lin SY, et al. Identification of MYST3 as a novel epigenetic activator of ERalpha frequently amplified in breast cancer. Oncogene. 2017;36:2910–8.
Sheikh BN, Phipson B, El-Saafin F, Vanyai HK, Downer NL, Bird MJ, et al. MOZ (MYST3, KAT6A) inhibits senescence via the INK4A-ARF pathway. Oncogene. 2015;34:5807–20.
Fei D, Wang Y, Zhai Q, Zhang X, Zhang Y, Wang Y, et al. KAT6A regulates stemness of aging bone marrow-derived mesenchymal stem cells through Nrf2/ARE signaling pathway. Stem Cell Res Ther. 2021;12:104.
Lv D, Jia F, Hou Y, Sang Y, Alvarez AA, Zhang W, et al. Histone acetyltransferase KAT6A upregulates PI3K/AKT signaling through TRIM24 binding. Cancer Res. 2017;77:6190–201.
Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L, et al. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma. Theranostics. 2018;8:4649–63.
Dou C, Liu Z, Tu K, Zhang H, Chen C, Yaqoob U, et al. P300 acetyltransferase mediates stiffness-induced activation of hepatic stellate cells into tumor-promoting myofibroblasts. Gastroenterology. 2018;154:2209–21.
Liu Z, Wang Y, Dou C, Sun L, Li Q, Wang L, et al. MicroRNA-1468 promotes tumor progression by activating PPAR-gamma-mediated AKT signaling in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:49.
Sun C, Sun L, Li Y, Kang X, Zhang S, Liu Y. Sox2 expression predicts poor survival of hepatocellular carcinoma patients and it promotes liver cancer cell invasion by activating Slug. Med Oncol. 2013;30:503.
Tsai WW, Wang Z, Yiu TT, Akdemir KC, Xia W, Winter S, et al. TRIM24 links a non-canonical histone signature to breast cancer. Nature. 2010;468:927–32.
Groner AC, Cato L, de Tribolet-Hardy J, Bernasocchi T, Janouskova H, Melchers D, et al. TRIM24 is an oncogenic transcriptional activator in prostate cancer. Cancer Cell. 2016;29:846–58.
Yang N, Chen T, Wang L, Liu R, Niu Y, Sun L, et al. CXCR4 mediates matrix stiffness-induced downregulation of UBTD1 driving hepatocellular carcinoma progression via YAP signaling pathway. Theranostics. 2020;10:5790–801.
Huang F. New KAT6 inhibitors induce senescence and arrest cancer growth. Synth Syst Biotechnol. 2018;3:244–5.
Turner-Ivey B, Guest ST, Irish JC, Kappler CS, Garrett-Mayer E, Wilson RC, et al. KAT6A, a chromatin modifier from the 8p11-p12 amplicon is a candidate oncogene in luminal breast cancer. Neoplasia. 2014;16:644–55.
Liu W, Zhan Z, Zhang M, Sun B, Shi Q, Luo F, et al. KAT6A, a novel regulator of beta-catenin, promotes tumorigenicity and chemoresistance in ovarian cancer by acetylating COP1. Theranostics. 2021;11:6278–92.
Saglam O, Tang Z, Tang G, Medeiros LJ, Toruner GA. KAT6A amplifications are associated with shorter progression-free survival and overall survival in patients with endometrial serous carcinoma. PloS ONE. 2020;15:e0238477.
Albhaisi S, Sanyal AJ. Applying non-invasive fibrosis measurements in NAFLD/NASH: progress to date. Pharmaceut Med. 2019;33:451–63.
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906.
Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205.
Li G, Chen S, Zhang Y, Xu H, Xu D, Wei Z, et al. Matrix stiffness regulates alpha-TAT1-mediated acetylation of alpha-tubulin and promotes silica-induced epithelial-mesenchymal transition via DNA damage. J Cell Sci. 2021;134:jcs243394.
Rokudai S, Laptenko O, Arnal SM, Taya Y, Kitabayashi I, Prives C. MOZ increases p53 acetylation and premature senescence through its complex formation with PML. Proc Natl Acad Sci USA. 2013;110:3895–900.
Yang XJ. MOZ and MORF acetyltransferases: molecular interaction, animal development and human disease. Biochim Biophys Acta. 2015;1853:1818–26.
Rokudai S, Aikawa Y, Tagata Y, Tsuchida N, Taya Y, Kitabayashi I. Monocytic leukemia zinc finger (MOZ) interacts with p53 to induce p21 expression and cell-cycle arrest. J Biol Chem. 2009;284:237–44.
Zhang LH, Yin YH, Chen HZ, Feng SY, Liu JL, Chen L, et al. TRIM24 promotes stemness and invasiveness of glioblastoma cells via activating Sox2 expression. Neuro Oncol. 2020;22:1797–808.
Xie W, Zhang Y, Wang B, Hu Y, Zhan B, Wei F, et al. Tripartite motif containing 24 regulates cell proliferation in colorectal cancer through YAP signaling. Cancer Med. 2020;9:6367–76.
Funding
This study was supported by grants from the National Natural Science Foundation of China (82103428), Natural Science Basic Research Program of Shaanxi (No.2020JQ-496, No.2020JQ-498); the Institutional Foundation of the First Affiliated Hospital of Xi’an Jiaotong University (2019QN-24, 2020QN-10); the Fundamental Research Funds for the Central Universities (Program No. xzy012020041).
Author information
Authors and Affiliations
Contributions
Study concept and design: ZKL and NY. Acquisition of the data: WZ, HYM, RKL, TXC and NY. Management of data acquisition: WZ, HYM, RKL, TXC, ZKL and NY. Analysis of the present data: WZ, HYM and ZKL. Statistical analysis: WZ and NY. Critical interpretation of the present data: WZ, HYM, RKL, TXC and NY. Drafting of the paper: WZ, ZKL and NY. Critical revision of the paper for important intellectual content: WZ, HYM, RKL, TXC, ZKL and NY. Obtained funding: ZKL and NY. Technical or material support: WZ, ZKL and NY. Study supervision: ZKL and NY.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments in our study were carried out in accordance with the Helsinki Declaration, and approved by the Ethics Committee the First Affiliated Hospital of Xi’an Jiaotong University (XJTU-2021-668). Patients were informed that the resected specimens were stored by the hospital and potentially used for scientific research, and that their privacy would be maintained. All patients provided informed consent prior to undergoing screening procedures. Our study protocol was approved by the Ethics Committee of Xi’an Jiaotong University (Xi’an, China).
Consent to publish
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, W., Mo, H., Liu, R. et al. Matrix stiffness-induced upregulation of histone acetyltransferase KAT6A promotes hepatocellular carcinoma progression through regulating SOX2 expression. Br J Cancer 127, 202–210 (2022). https://doi.org/10.1038/s41416-022-01784-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01784-9
This article is cited by
-
MORF and MOZ acetyltransferases target unmethylated CpG islands through the winged helix domain
Nature Communications (2023)
-
miR-339-3p inhibits cell growth and epithelial–mesenchymal transition in nasopharyngeal carcinoma by modulating the KAT6A/TRIM24 axis
International Journal of Clinical Oncology (2022)