Abstract
Background
To estimate the impact of oophorectomy and other treatments on the survival of breast cancer patients with a CHEK2 mutation.
Methods
Women with Stage I–III breast cancer who were treated at 17 hospitals in Poland were tested for four founder mutations in the CHEK2 gene. 974 women (10%) were positive for a CHEK2 mutation. Control patients without a CHEK2 mutation were selected from a database of patients treated over the same time period. Information on treatments received and distant recurrences were retrieved from medical records. Treatments included chemotherapy, hormonal therapy (tamoxifen) and radiation therapy. Oophorectomies were performed for the treatment of breast cancer or for benign conditions. Dates of death were obtained from the Polish Vital Statistics Registry. Causes of death were determined by medical record review. Predictors of survival were determined using the Cox proportional hazards model.
Results
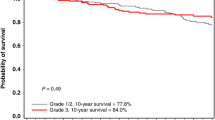
In all, 839 patients with a CHEK2 mutation were matched to 839 patients without a mutation. The mean follow-up was 12.0 years. The 15-year survival for CHEK2 carriers was 76.6% and the 15-year survival for non-carrier control patients was 78.8% (adjusted HR = 1.06; 95% CI: 0.84–1.34; P = 0.61). Among CHEK2 carriers, the 15-year survival for women who had an oophorectomy was 86.3% and for women who did not have an oophorectomy was 72.1% (adjusted HR = 0.59; 95% CI: 0.38–0.90; P = 0.02). Among controls, the 15-year survival for patients who had an oophorectomy was 84.5% and for women who did not have an oophorectomy was 77.6% (adjusted HR = 1.03; 95% CI: 0.66–1.61; P = 0.90).
Conclusion
Among women with breast cancer and a CHEK2 mutation, oophorectomy is associated with a reduced risk of death from breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data relevant to the study are included in the article.
References
Cybulski C, Kluźniak W, Huzarski T, Wokołorczyk D, Kashyap A, Jakubowska A, et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. Lancet Oncol. 2015;16:638–44.
Jonasson JG, Stefansson OA, Johannsson OT, Sigurdsson H, Agnarsson BA, Olafsdottir GH, et al. Oestrogen receptor status, treatment and breast cancer prognosis in Icelandic BRCA2 mutation carriers. Br J Cancer. 2016;115:776–83.
Olafsdottir EJ, Borg A, Jensen MB, Gerdes AM, Johansson ALV, Barkardottir RB, et al. Breast cancer survival in Nordic BRCA2 mutation carriers-unconventional association with oestrogen receptor status. Br J Cancer. 2020;123:1608–15.
Evans DG, Phillips KA, Milne R, Fruscio R, Cybulski C, Gronwald J, et al. Survival from breast cancer in women with a BRCA2 mutation by treatment. Br J Cancer. 2021;124:1524–32.
Narod SA, Huzarski T, Gronwald J, Byrski T, Marczyk E, Cybulski C, et al. Predictors of survival for breast cancer patients with a BRCA1 mutation. Breast Cancer Res Treat. 2018;168:513–21.
Metcalfe K, Lynch HT, Foulkes WD, Tung N, Kim-Sing C, Olopade OI, et al. Effect of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. JAMA Oncol. 2015;1:306–13.
Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37.
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29:3739–46.
Gordhandas S, Norquist BM, Pennington KP, Yung RL, Laya MB, Swisher EM. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192–200.
Holmberg L, Anderson H. HABITS steering and data monitoring committees. HABITS (hormonal replacement therapy after breast cancer-is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363:453–5.
Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat. Genet. 2002;31:55–59.
Hu C, Hart SN, Gnanaolivu R, Huang H, Lee KY, Na J, et al. A population-based study of genes previously implicated in breast cancer. N. Engl J Med. 2021;384:440–51.
Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C, et al. Breast cancer risk genes - association analysis in more than 113,000 women. N. Engl J Med. 2021;384:428–39.
Narod SA. Which genes for hereditary breast cancer? N. Engl J Med. 2021;384:471–3.
Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–7.
Cybulski C, Wokołorczyk D, Jakubowska A, Huzarski T, Byrski T, Gronwald J, et al. Risk of breast cancer in women with a CHEK2 mutation with and without a family history of breast cancer. J Clin Oncol. 2011;29:3747–52.
Cybulski C, Wokołorczyk D, Huzarski T, Byrski T, Gronwald J, Górski B, et al. A deletion in CHEK2 of 5,395 bp predisposes to breast cancer in Poland. Breast Cancer Res Treat. 2007;102:119–22.
Cybulski C, Huzarski T, Byrski T, Gronwald J, Debniak T, Jakubowska A, et al. Estrogen receptor status in CHEK2-positive breast cancers: implications for chemoprevention. Clin Genet. 2009;75:72–8.
Cybulski C, Kluźniak W, Huzarski T, Wokołorczyk D, Kashyap A, Rusak B, et al. The spectrum of mutations predisposing to familial breast cancer in Poland. Int J Cancer. 2019;145:3311–20.
Weischer M, Bojesen SE, Ellervik C, Tybjaerg-Hansen A, Nordestgaard BG. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analysis of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26:542–8.
Weischer M, Nordestgaard BG, Pharoah P, Bolla MK, Nevanlinna H, Van’t Veer LJ, et al. CHEK2*1100delC heterozygosity in women with breast cancer associated with early death, breast cancer-specific death, and increased risk of a second breast cancer. J Clin Oncol. 2012;30:4308–16.
Muranen TA, Blomqvist C, Dörk T, Jakubowska A, Heikkilä P, Fagerholm R, et al. Patient survival and tumor characteristics associated with CHEK2:p.I157T - findings from the Breast Cancer Association Consortium. Breast Cancer Res. 2016;18:98.
Kriege M, Hollestelle A, Jager A, Huijts PE, Berns EM, Sieuwerts AM, et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: impact of adjuvant chemotherapy. Br J Cancer. 2014;111:1004–13.
Greville-Heygate SL, Maishman T, Tapper WJ, Cutress RI, Copson E, Dunning AM, et al. Pathogenic variants in CHEK2 are associated with an adverse prognosis in symptomatic early-onset breast cancer. JCO Precis Oncol. 2020;4:PO.19.00178.
Schmidt MK, Tollenaar RA, de Kemp SR, Broeks A, Cornelisse CJ, Smit VT, et al. Breast cancer survival and tumor characteristics in premenopausal women carrying the CHEK2*1100delC germline mutation. J Clin Oncol. 2007;25:64–69.
Pfeifer W, Sokolenko AP, Potapova ON, Bessonov AA, Ivantsov AO, Laptiev SA, et al. Breast cancer sensitivity to neoadjuvant therapy in BRCA1 and CHEK2 mutation carriers and non-carriers. Breast Cancer Res Treat. 2014;148:675–83.
Liu Y, Xu Y, Ouyang T, Li J, Wang T, Fan Z, et al. Association between CHEK2 H371Y mutation and response to neoadjuvant chemotherapy in women with breast cancer. BMC Cancer. 2015;15:194.
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, et al. TBCRC 048: phase II study of Olaparib for metastatic breast cancer and mutations in homologous recombination-related Genes. J Clin Oncol. 2020;38:4274–82.
Knappskog S, Chrisanthar R, Løkkevik E, Anker G, Østenstad B, Lundgren S, et al. Low expression levels of ATM may substitute for CHEK2/TP53 mutations predicting resistance towards anthracycline and mitomycin chemotherapy in breast cancer. Breast Cancer Res. 2012;14:R47.
Huzarski T, Cybulski C, Wokolorczyk D, Jakubowska A, Byrski T, Gronwald J, et al. Survival from breast cancer in patients with CHEK2 mutations. Breast Cancer Res Treat. 2014;144:397–403.
Funding
The work described in this article was funded by the Polish Ministry of Health. Steven Narod is a recipient of a Canada Research Chair Tier 1 and is supported by the Peter Gilgan Center for Women’s Cancers at Women’s College Hospital.
Author information
Authors and Affiliations
Consortia
Contributions
JT-S, MS and MF reviewed clinical information prepared the data files and conducted quality control. CC, DW and AJ conducted the laboratory analyses. JG, MS, DG, EK, EM, MS, RW, OH, RS and LB submitted clinical data and performed the medical review. PS and SAN performed the statistical analysis and edited the paper. JL and TH conceived of the paper and co-ordinated the collaborative group.
Corresponding author
Ethics declarations
Competing interests
SAN is an editorial board member of BJC. The remaining authors declare no competing interests.
Ethics approval and consent to participate
The study is exempt from informed patient consent and board approval by the Women’s College Hospital Ethics Board. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
Individual personal data are not included.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prof. Jan Lubinski is the nominated Consortium representative.
Supplementary information
Rights and permissions
About this article
Cite this article
Tomiczek-Szwiec, J., Szwiec, M., Falco, M. et al. The impact of oophorectomy on survival from breast cancer in patients with CHEK2 mutations. Br J Cancer 127, 84–91 (2022). https://doi.org/10.1038/s41416-022-01770-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01770-1
This article is cited by
-
Genetic testing for hereditary breast cancer in Poland: 1998–2022
Hereditary Cancer in Clinical Practice (2023)