Abstract
Background
Bone-marrow-derived haematopoietic stem and progenitor cells (HSPCs) are a prominent part of the highly complex tumour microenvironment (TME) where they localise within tumours and maintain haematopoietic potency. Understanding the role HSPCs play in tumour growth and response to radiation therapy (RT) may lead to improved patient treatments and outcomes.
Methods
We used a mouse model of non-small cell lung carcinoma where tumours were exposed to RT regimens alone or in combination with GW2580, a pharmacological inhibitor of colony stimulating factor (CSF)-1 receptor. RT-PCR, western blotting and immunohistochemistry were used to quantify expression levels of factors that affect HSPC differentiation. DsRed+ HSPC intratumoural activity was tracked using flow cytometry and confocal microscopy.
Results
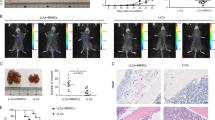
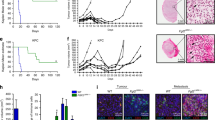
We demonstrated that CSF-1 is enhanced in the TME following exposure to RT. CSF-1 signaling induced intratumoural HSPC differentiation into M2 polarised tumour-associated macrophages (TAMs), aiding in post-RT tumour survival and regrowth. In contrast, hyperfractionated/pulsed radiation therapy (PRT) and GW2580 ablated this process resulting in improved tumour killing and mouse survival.
Conclusions
Tumours coopt intratumoural HSPC fate determination via CSF-1 signaling to overcome the effects of RT. Thus, limiting intratumoural HSPC activity represents an attractive strategy for improving the clinical treatment of solid tumours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are included in this published article and are available (GJM) upon reasonable request.
References
Kane J, Krueger SA, Dilworth JT, Torma JT, Wilson GD, Marples B, et al. Hematopoietic stem and progenitor cell migration after hypofractionated radiation therapy in a murine model. Int J Radiat Oncol Biol Phys. 2013;87:1162–70.
Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9.
Liu Y, Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. 2015;12:1–4.
Kim J, Bae JS. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediators Inflamm. 2016;2016:6058147.
Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65.
Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12.
Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4:376–89.
Jeannin P, Paolini L, Adam C, Delneste Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 2018;285:680–99.
Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Macrophages within NSCLC tumour islets are predominantly of a cytotoxic M1 phenotype associated with extended survival. Eur Respir J. 2009;33:118–26.
Chung FT, Lee KY, Wang CW, Heh CC, Chan YF, Chen HW, et al. Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer. 2012;131:E227–235.
Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5:1507–15.
Zhang B, Yao G, Zhang Y, Gao J, Yang B, Rao Z, et al. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo). 2011;66:1879–86.
Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60.
Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20.
Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 2007;67:11687–95.
Bigoni-Ordonez GD, Czarnowski D, Parsons T, Madlambayan GJ, Villa-Diaz LG. Integrin alpha6 (CD49f), the microenvironment and cancer stem cells. Curr Stem Cell Res Ther. 2019;14:428–36.
Jourdan MM, Lopez A, Olasz EB, Duncan NE, Demara M, Kittipongdaja W, et al. Laminin 332 deposition is diminished in irradiated skin in an animal model of combined radiation and wound skin injury. Radiat Res. 2011;176:636–48.
Cristofaro B, Emanueli C. Possible novel targets for therapeutic angiogenesis. Curr Opin Pharmacol. 2009;9:102–8.
Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506.
Dilworth JT, Krueger SA, Dabjan M, Grills IS, Torma J, Wilson GD, et al. Pulsed low-dose irradiation of orthotopic glioblastoma multiforme (GBM) in a pre-clinical model: effects on vascularization and tumor control. Radiother Oncol. 2013;108:149–54.
Kane JL, Krueger SA, Hanna A, Raffel TR, Wilson GD, Madlambayan GJ, et al. Effect of irradiation on tumor microenvironment and bone marrow cell migration in a preclinical tumor model. Int J Radiat Oncol Biol Phys. 2016;96:170–8.
Almahariq MF, Quinn TJ, Arden JD, Roskos PT, Wilson GD, Marples B, et al. Pulsed radiation therapy for the treatment of newly diagnosed glioblastoma. Neuro Oncol. 2020. https://doi.org/10.1093/neuonc/noaa165.
Madlambayan GJ, Butler JM, Hosaka K, Jorgensen M, Fu D, Guthrie SM, et al. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114:4310–9.
Iyer G, Wang AR, Brennan SR, Bourgeois S, Armstrong E, Shah P, et al. Identification of stable housekeeping genes in response to ionizing radiation in cancer research. Sci Rep. 2017;7:43763.
A Package for Survival Analysis in R [computer program]. Version 3.2-7. 2020. https://CRAN.R-project.org/package=survival2020.
Arndt K, Grinenko T, Mende N, Reichert D, Portz M, Ripich T, et al. CD133 is a modifier of hematopoietic progenitor frequencies but is dispensable for the maintenance of mouse hematopoietic stem cells. Proc Natl Acad Sci USA. 2013;110:5582–7.
Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE. 2015;10:e0145342.
Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71.
Hu Y, He MY, Zhu LF, Yang CC, Zhou ML, Wang Q, et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35:12.
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602.
Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch’ng ES. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol. 2019;9:1512.
Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36:229–39.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72.
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69.
Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705.
Szilvassy SJ, Meyerrose TE, Ragland PL, Grimes B. Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood. 2001;98:2108–15.
Acknowledgements
The BRI animal facility staff and SG assisted with animal husbandry and tumour inoculations.
Funding
This work was supported by an OU Center for Biomedical Research grant (GJM) and OU Provost grant (TMP). Flow cytometry was performed in the OU Flow Cytometry Core, supported in part by a NSF-MRI (NSF-1919572) grant (LGV-D and GJM).
Author information
Authors and Affiliations
Contributions
GJM, GW, LGV-D and TMP conceived the project. TMP, KLB, AH and CP performed the experiments. TMP, KLB, MAB, SEH, RJW, LGV-D, GW and GJM provided resources and analysed data. TMP, GW, LGV-D and GJM were responsible for project management and compliance protocols. GJM, TMP, SEH, RJW and LGV-D wrote the paper. GW and KLB edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal experiments were approved by the BRI IACUC.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Parsons, T.M., Buelow, K.L., Hanna, A. et al. Intratumoural haematopoietic stem and progenitor cell differentiation into M2 macrophages facilitates the regrowth of solid tumours after radiation therapy. Br J Cancer 126, 927–936 (2022). https://doi.org/10.1038/s41416-021-01652-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01652-y
This article is cited by
-
Prediction of hub genes and key pathways associated with the radiation response of human hematopoietic stem/progenitor cells using integrated bioinformatics methods
Scientific Reports (2023)
-
LARRPM restricts lung adenocarcinoma progression and M2 macrophage polarization through epigenetically regulating LINC00240 and CSF1
Cellular & Molecular Biology Letters (2022)