Abstract
Background
Colorectal cancer (CRC) patients have a better prognosis if metastases are resectable. Initially, unresectable liver-only metastases can be converted to resectable with chemotherapy plus a targeted therapy. We assessed which of chemotherapy doublet (2-CTx) or triplet (3-CTx), combined with targeted therapy by RAS status, would be better in this setting.
Methods
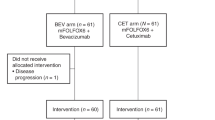
PRODIGE 14 was an open-label, multicenter, randomised Phase 2 trial. CRC patients with initially defined unresectable liver-only metastases received either, 2-CTx (FOLFOX or FOLFIRI) or 3-CTx (FOLFIRINOX), plus bevacizumab/cetuximab by RAS status. The primary endpoint was to increase the R0/R1 liver-resection rate from 50 to 70% with the 3-CTx.
Results
Patients (n = 256) were mainly men with an ECOG PS of 0, and a median age of 60 years. In total, 109 patients (42.6%) had RAS-mutated tumours. After a median follow-up of 45.6 months, the R0/R1 liver-resection rate was 56.9% (95% CI: 48–66) with the 3-CTx versus 48.4% (95% CI: 39–57) with the 2-CTx (P = 0.17). Median overall survival was 43.4 months with 3-CTx versus 40 months with 2-CTx.
Conclusion
We failed to increase from 50 to 70% the R0/R1 liver-resection rate with the use of 3-CTx combined with bevacizumab or cetuximab by RAS status in CRC patients with initially unresectable liver metastases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Unicancer will share de-identified individual data that underlie the results reported under the following conditions: the data shared will be limited to that required for independent mandated verification of the published results, the reviewer will need authorisation from Unicancer for personal access, and data will only be transferred after signing of a data access agreement. A decision concerning the sharing of other study documents, including protocol and statistical analysis plan, will be examined upon request. Unicancer will consider access to study data upon written detailed request sent to l-monard@unicancer.fr, from 6 months until 5 years after the publication of this article.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–8.
Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702–8.
Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. J Am Med Assoc. 2017;317:2392–2401.
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931–8.
Kohne CH, Poston G, Folprecht G, Ciardiello F, Ronga P, Beier F, et al. FOLFIRI plus cetuximab in patients with liver-limited or non-liver-limited RAS wild-type metastatic colorectal cancer: a retrospective subgroup analysis of the CRYSTAL study. Eur J Surg Oncol. 2016;42:1540–7.
Leone F, Artale S, Marino D, Cagnazzo C, Cascinu S, Pinto C, et al. Panitumumab in combination with infusional oxaliplatin and oral capecitabine for conversion therapy in patients with colon cancer and advanced liver metastases. The MetaPan study. Cancer. 2013;119:3429–35.
Wang L, Sun Y, Zhao B, Zhang H, Yu Q, Yuan X. Chemotherapy plus targeted drugs in conversion therapy for potentially resectable colorectal liver metastases: a meta-analysis. Oncotarget. 2016;7:55732–40.
Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–9.
Okuno M, Hatano E, Nishino H, Seo S, Taura K, Uemoto S. Does response rate of chemotherapy with molecular target agents correlate with the conversion rate and survival in patients with unresectable colorectal liver metastases?: a systematic review. Eur J Surg Oncol. 2017;43:1003–1012.
Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, et al. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol. 2013;20:4289–97.
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–6.
Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G, et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103:21–30.
Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–52.
Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl J Med. 2014;371:1609–17.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–15.
Cremolini C, Casagrande M, Loupakis F, Aprile G, Bergamo F, Masi G, et al. Efficacy of FOLFOXIRI plus bevacizumab in liver-limited metastatic colorectal cancer: a pooled analysis of clinical studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer. 2017;73:74–84.
Assenat E, Desseigne F, Thezenas S, Viret F, Mineur L, Kramar A, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16:1557–64.
Cremolini C, Antoniotti C, Lonardi S, Aprile G, Bergamo F, Masi G, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. 2018;4:529–536.
Geissler M, Riera-Knorrenschild J, Tannapfel A, Greeve J, Florschütz A, Wessendorf S, et al. mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized phase II VOLFI trial of the AIO (AIO- KRK0109). J Clin Oncol. 2018;36:3509–3509.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
Phelip JM, Tougeron D, Léonard D, Benhaim L, Desolneux G, Dupré A, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2019;51:1357–1363.
Ychou M, Viret F, Kramar A, Desseigne F, Mitry E, Guimbaud R, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharm. 2008;62:195–201.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl J Med. 2011;364:1817–25.
Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wie AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl J Med. 2018;379:2395–2406.
Acknowledgements
We thank the patients and their families for participating in the study. Also, we would like to thank Trevor Stanbury (UNICANCER) for medical writing support. We are also indebted to all the participating centers and to The Ligue Nationale Contre le Cancer. This trial was made possible through an unrestricted grant from Merck-Serono France.
Funding
This trial was made possible through an unrestricted grant from Merck-Serono France. Investigators list: INVESTIGATOR NAME: SITE, CITY. Pr. Marc YCHOU: Institut Régional du Cancer de Montpellier/Val d’Aurelle (ICM), Montpellier; Dr. Laurent MINEUR: Institut Ste Catherine, Avignon; Pr. Olivier BOUCHE: CHU de Reims, Reims; Dr. Pascale MARIANI: Institut Curie, Paris; Dr. Kartine BOUHIER-LEPORIER: CHU Côte de Nacre, Caen; Dr. Marianne FONCK: Institut Bergonié, Bordeaux; Dr. Roger FAROUX: CHD Vendée, La Roche sur Yon; Dr. Alice GAGNAIRE: CHU Dijon - Hôpital Du Bocage, Dijon; Dr. Eric FRANCOIS: Centre Antoine Lacassagne, Nice; Dr. Julien FORESTIER: Hôpital Edouard Herriot, Lyon; Pr François GHIRINGHELLI: Centre G. F. Leclerc, Dijon; Dr. Driffa MOUSSATA: Centre hospitalier Lyon Sud, Pierre-Benite; Dr. Gaël DEPLANQUE: Centre Hospitalier Saint-Joseph, Paris; Dr. Jean-Louis LEGOUX: CHR d’Orléans - La Source, Orleans; Dr. Cédric LECAILLE: Polyclinique de Bordeaux Nord Aquitaine, Bordeaux; Pr. Nicole TUBIANA-MATHIEU: CHU Dupuytren, Limoges; Mr. Michel RIVOIRE: Centre Léon Bérard, Lyon; Dr. Marie-Pierre GALAIS: Centre François Baclesse, Caen; Pr. Rosine GUIMBAUD: Centre hospitalier Rangueil, Toulouse; Dr. Francine FEIN: CHU Jean Minjoz, Besancon; Dr. Philippe HOUYAU: Clinique Claude Bernard, Albi; Pr. Thomas APARICIO: Hôpital Avicenne, Bobigny; Pr. Jean-François SEITZ: CHU La Timone, Marseille; Dr. Stephen ELLIS: Centre Catalan d’oncologie, Perpignan; Dr. Stéphane REMY: Centre d’oncologie et de radiothérapie du Pays basque, Bayonne; Dr. Faiza KHEMISSA - AKOUZ: Hôpital Saint Jean, Perpignan; Dr. Mohammed RAMDANI: Centre Hospitalier de Béziers, Béziers; Dr. Anne MERCIER - BLAS: CHP Saint Grégoire, Saint Gregoire; Pr. Eric ASSENAT: CHU Saint Eloi - CHRU de Montpellier, Montpellier; Pr. Julien TAIEB: Hôpital Européen Georges Pompidou, Paris; Pr. René ADAM: Hôpital Paul Brousse, Villejuif; Dr. Hervé PERRIER: Hôpital St Joseph, Marseille; Dr. Agnès PELAQUIER: Centre hospitalier Montelimar, Montelimar; Pr. Emmanuel MITRY: Hôpital Huguenin - Institut Curie, Saint-Cloud; Dr. Serge FRATTE: CHBM - Site du Mittan, Montbeliard; Dr. Franck AUDEMAR: Centre hospitalier Côte Basque, Bayonne; Dr. Jean-Luc LABOUREY: Centre hospitalier de Carcassonne, Carcassone; Dr. Mathilde MARTINEZ: Clinique Pasteur, Toulouse; Dr. Christian BOREL: Centre Paul Strauss, Strasbourg; Dr. Etienne SUC: Clinique St Jean du Languedoc, Toulouse; Dr. Christine LE FOLL: Centre hospitalier Marne la Vallée, Jossigny; Dr. Etienne MAURIAC: Polyclinique Côte Basque Sud, Saint Jean de Luz; Dr. Denis PÈRE-VERGĒ: Centre Hospitalier Saint-Joseph-Saint-Luc, Lyon.
Author information
Authors and Affiliations
Contributions
Dr. MY had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: MY, RA, OB and MR. Acquisition, analysis or interpretation of the data: MY, AA and ST. Drafting of the manuscript: AA, EL-C, ST and MY. Critical revision of the manuscript for important intellectual content: all the authors. Statistical analysis: Thezenas. Administrative, technical or material support: MY, MR, RG, FG, EL-C, AM-B, LM, EF, FK, MC, RK, MF, PH, TA, M-PG, FA, EA, CJ, AA and OB; supervision: MY.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki, good clinical practice guidelines, and other local laws. The study documents were approved by a French ethics committee, “Comité de Protection des Personnes Sud Méditerranée IV”. Patients provided written informed consent before enrolment.
Consent to publish
Not applicable.
Competing interests
MY reported receiving honoraria from Amgen, Bayer, Merck, Roche, and Servier. MR has nothing to disclose. ST has nothing to disclose. RG reported personal fees from AAA, Amgen, Astra-Zeneca, BMS, P. Fabre, Novartis, Roche, Servier, outside the submitted work. FG served on external advisory boards for Roche; research funding from Roche, Genentech, Amgen, Enterome, Servier; received funding for a clinical trial from Astra-Zeneca, received fee for communication by Amgen, Astra-Zeneca, BMS, Sanofi, Merck-Serono, Servier and received fee for travel by Roche and Servier. AM-B has nothing to disclose. LM has nothing to disclose. EF reported personal fees from ROCHE, personal fees from SERVIER, personal fees from NOVARTIS, personal fees from MSD, outside the submitted work. FK reported personal feed from Sanofi, other fees from Roche (congress support), other fees from Ipsen (congress support), outside the submitted work. MC has nothing to disclose. RK has nothing to disclose. MF has nothing to disclose. PH has nothing to disclose. TA reported personal fees from Roche, personal fees from Servier, personal fees from Amgen, personal fees from Ipsen, personal fees from Sanofi, non-financial support from Bayer, outside the submitted work. M-PG reported other relevant financial interest from Roche (travel), other relevant financial interest from Amgen (travel, board), outside the submitted work. FA reported personal fees from Sanofi, personal fees from Merck, personal fees from Amgen, personal fees from Servier, personal fees from Roche, outside the submitted work. EA reported other fees (advisory board) from Roche, from Astrazeneca, from Ipsen, from Bayer, from Sanofi, from AMGEN, from AAA, outside the submitted work. EL-C has nothing to disclose, CJ has nothing to disclose. AA reported receiving honoraria from Bayer, Bristol-Myers Squibb, Merck-Sharp Dohme, Sanofi, and Servier. RA reported personal fees from Merck (congress presentation), personal fees from Sanofi (congress presentation), outside the submitted work. OB reported personal fees from ROCHE (self honoraria, advisory/consultancy), personal fees from AMGEN (self honoraria, speaker bureau/expert testimony), personal fees from MERCK KGaA (self honoraria, advisory/consultancy), personal fees from SERVIER (self honoraria, speaker bureau/expert testimony), personal fees from BAYER (self honoraria, advisory/consultancy), personal fees from PIERRE FABRE (self honoraria, speaker bureau/expert testimony), personal fees from Astra-Zeneca (self honoraria, advisory/consultancy), personal fees from Grunenthal (self honoraria, advisory/consultancy), personal fees from MSD (Self honoraria, advisory/consultancy), non-financial support from Roche (travel/accommodation/expenses) and non-financial support from Servier (travel/accommodation/expenses) outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ychou, M., Rivoire, M., Thezenas, S. et al. Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer 126, 1264–1270 (2022). https://doi.org/10.1038/s41416-021-01644-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01644-y
This article is cited by
-
The efficacy and safety of anti-EGFR target agents in patients with potentially resectable metastatic colorectal cancer: a meta-analysis of randomized controlled trials
World Journal of Surgical Oncology (2023)
-
Oligometastatic Colorectal Cancer: A Review of Definitions and Patient Selection for Local Therapies
Journal of Gastrointestinal Cancer (2023)