Abstract
Background
Cabozantinib is an oral tyrosine kinase inhibitor in renal cell carcinoma (RCC), whose targets include oncogenic AXL and unique ligand GAS6. Critical gaps in basic knowledge need to be addressed to devise an exclusive biomarker and candidate when targeting the AXL/GAS6 axis.
Methods
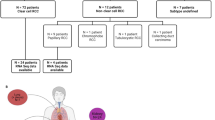
To clarify the effects of the AXL/GAS6 axis on RCC, we herein performed a large-scale immunogenomic analysis and single-cell counts including various metastatic organs and histological subtypes of RCC. We further applied genome-wide mutation analyses and methylation arrays.
Results
Varying patterns of AXL and GAS6 expression were observed throughout primary RCC tumours and metastases. Scoring individual AXL/GAS6 levels in the tumour centre and invasive margin, namely, the AXL/GAS6 score, showed a good ability to predict the prognosis of clear cell RCC. Metastasis- and histological subtype-specific differences in the AXL/GAS6 score existed since lung metastasis and the papillary subtype were weakly related to the AXL/GAS6 axis. Cell-by-cell immunohistological assessments clarified an immunosuppressive environment in tumours with high AXL/GAS6 scores. Genomic alterations in the PI3K-mTOR pathway and DNA methylation profiling revealed distinct differences with the AXL/GAS6 score in ccRCC.
Conclusion
The AXL/GAS6 scoring system could predict the outcome of prognosis and work as a robust biomarker for the immunogenomic state in RCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
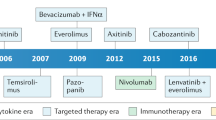
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N. Engl J Med. 2015;373:1814–23.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–27.
Escudier B, Powles T, Motzer RJ, Olencki T, Aren Frontera O, Oudard S, et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J Clin Oncol. 2018;36:765–72.
Cella D, Escudier B, Tannir NM, Powles T, Donskov F, Peltola K, et al. Quality of life outcomes for cabozantinib versus everolimus in patients with metastatic renal cell carcinoma: METEOR phase III randomized trial. J Clin Oncol. 2018;36:757–64.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl J Med. 2015;373:1803–13.
Cella D, Grunwald V, Nathan P, Doan J, Dastani H, Taylor F, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:994–1003.
Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35:591–7.
Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl J Med. 2018;378:1277–90.
Motzer RJ, Rini BI, McDermott DF, Aren Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20:1370–85.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl J Med. 2019;380:1116–27.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl J Med. 2019;380:1103–15.
Rini BI, Battle D, Figlin RA, George DJ, Hammers H, Hutson T, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J Immunother Cancer. 2019;7:354.
Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799–810.
Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:706–20.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9.
Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:6352.
Shen Y, Chen X, He J, Liao D, Zu X. Axl inhibitors as novel cancer therapeutic agents. Life Sci. 2018;198:99–111.
Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18:153.
Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–97.
Brown M, Black JR, Sharma R, Stebbing J, Pinato DJ. Gene of the month: Axl. J Clin Pathol. 2016;69:391–7.
Aguilera TA, Giaccia AJ. Molecular pathways: oncologic pathways and their role in T-cell exclusion and immune evasion—a new role for the AXL receptor tyrosine kinase. Clin Cancer Res. 2017;23:2928–33.
Flaifel A, Xie W, Braun DA, Ficial M, Bakouny Z, Nassar AH, et al. PD-L1 expression and clinical outcomes to cabozantinib, everolimus, and sunitinib in patients with metastatic renal cell carcinoma: analysis of the randomized clinical trials METEOR and CABOSUN. Clin Cancer Res. 2019;25:6080–8.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94.
Bergerot P, Lamb P, Wang E, Pal SK. Cabozantinib in combination with immunotherapy for advanced renal cell carcinoma and urothelial carcinoma: rationale and clinical evidence. Mol Cancer Ther. 2019;18:2185–93.
Watanabe K, Kosaka T, Aimono E, Hongo H, Mikami S, Nishihara H, et al. Japanese case of enzalutamide-resistant prostate cancer harboring a SPOP mutation with scattered allelic imbalance: response to platinum-based therapy. Clin Genitourin Cancer. 2019;17:e897–e902.
Naumova OY, Lee M, Koposov R, Szyf M, Dozier M, Grigorenko EL. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Dev Psychopathol. 2012;24:143–55.
Gunawardhana LP, Baines KJ, Mattes J, Murphy VE, Simpson JL, Gibson PG. Differential DNA methylation profiles of infants exposed to maternal asthma during pregnancy. Pediatr Pulmonol. 2014;49:852–62.
Martinez Chanza N, Xie W, Asim Bilen M, Dzimitrowicz H, Burkart J, Geynisman DM, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. 2019;20:581–90.
Schlumberger M, Elisei R, Muller S, Schoffski P, Brose M, Shah M, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann Oncol. 2017;28:2813–9.
Kelley RK, Verslype C, Cohn AL, Yang TS, Su WC, Burris H, et al. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol. 2017;28:528–34.
Goyette MA, Duhamel S, Aubert L, Pelletier A, Savage P, Thibault MP, et al. The receptor tyrosine kinase AXL is required at multiple steps of the metastatic cascade during HER2-positive breast cancer progression. Cell Rep. 2018;23:1476–90.
Sadahiro H, Kang KD, Gibson JT, Minata M, Yu H, Shi J, et al. Activation of the receptor tyrosine kinase AXL regulates the immune microenvironment in glioblastoma. Cancer Res. 2018;78:3002–13.
Acknowledgements
This study was supported by the Grant-in-Aid for Scientific Research (KAKENHI 20K18125 to KH; 18H04906, 18K19482 and 19H03792 to NT; 18K09150 to TS and 18H02939 to MO), the Takeda Science Foundation (NT), the Kobayashi Foundation for Cancer Research (NT), the SGH Cancer Research Grant (NT) and the Keio Gijuku Academic Development Funds (NT).
Author information
Authors and Affiliations
Contributions
NT, RM and MO designed the study. KH, KT, RT, YY and SM performed the experiments. EA and HN performed genome sequencing. TS, KK, TK, FM and TT provided conceptual advice. KH and NT wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures were performed in approval of the Research Ethics Committee of Keio University (Approval Nos.: 20180098 and 20190059) and in compliance with the 1964 Helsinki Declaration and present ethical standards. The samples were residual from a clinical examination without using any identifiable information of the individuals or the application of any intervention. Participation in the study was optional. Both written informed consent and passive (opt-out) informed consent procedures have been applied to the experimental use of human samples. Opt-out informed consent from patients was obtained by exhibiting the research information on our department website (Department of Urology, Keio University Hospital, Tokyo, Japan). All participant patients or families of deceased patients could withdraw consent by contacting the researcher with a 24-h phone number. The need to obtain written informed consent was waived if patients had finished their follow-up or had died, due to the study’s observational nature and the urgent need for cancer patient care. This was approved and reviewed by the Research Ethics Committee of Keio University, in accordance with the ethical guidelines for Medical and Health Research Involving Human Subjects (Public Notice of the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare as of July 2018; https://www.lifescience.mext.go.jp/files/pdf/n2181_01.pdf).
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hakozaki, K., Tanaka, N., Takamatsu, K. et al. Landscape of prognostic signatures and immunogenomics of the AXL/GAS6 axis in renal cell carcinoma. Br J Cancer 125, 1533–1543 (2021). https://doi.org/10.1038/s41416-021-01559-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01559-8
This article is cited by
-
Identification of genomic drivers for the therapeutic response of Cabozantinib in patients with metastatic renal cell carcinoma
World Journal of Urology (2024)
-
TAM family kinases as therapeutic targets at the interface of cancer and immunity
Nature Reviews Clinical Oncology (2023)
-
Prognostic role of the innate immune signature CD163 and “eat me” signal calreticulin in clear cell renal cell carcinoma
Cancer Immunology, Immunotherapy (2023)