Abstract
Background
We previously reported that CEA kinetics are a marker of progressive disease (PD) in metastatic colorectal cancer (mCRC). This study was specifically designed to confirm CEA kinetics for predicting PD and to evaluate CA19-9, cell-free DNA (cfDNA), circulating tumour DNA (ctDNA) and circulating tumour cell (CTC) kinetics.
Methods
Patients starting a chemotherapy (CT) with pre-treatment CEA > 5 ng/mL and/or CA19.9 > 30 UI/mL were prospectively included. Samples were collected from baseline to cycle 4 for CEA and CA19-9 and at baseline and the sixth week for other markers. CEA kinetics were calculated from the first to the third or fourth CT cycle.
Results
A total of 192 mCRC patients were included. CEA kinetics based on the previously identified >0.05 threshold was significantly associated with PD (p < 0.0001). By dichotomising by the median value, cfDNA, ctDNA and CA19-9 were associated with PD, PFS and OS in multivariate analysis. A circulating scoring system (CSS) combining CEA kinetics and baseline CA19-9 and cfDNA values classified patients based on high (n = 58) and low risk (n = 113) of PD and was independently associated with PD (ORa = 4.6, p < 0.0001), PFS (HRa = 2.07, p < 0.0001) and OS (HRa = 2.55, p < 0.0001).
Conclusions
CEA kinetics alone or combined with baseline CA19-9 and cfDNA are clinically relevant for predicting outcomes in mCRC.
Trial registration number
NCT01212510.
Similar content being viewed by others
Background
The algorithm for the treatment in metastatic colorectal cancer (mCRC) is complex with numerous options that depend mainly on the patient’s condition and tumour biology.1,2 To date, radiological examination using Response Evaluation Criteria In Solid Tumors (RECIST) is considered the standard for tumour assessment during treatment.3 In this context, circulating markers, such as carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), cell-free DNA (cfDNA), circulating tumour DNA (ctDNA) and circulating tumour cell (CTC), are considered very promising biomarkers to optimise patient decision making.4 Indeed, these markers are recognised as biologically relevant because they are released in the bloodstream, may reflect tumour dynamics, and harbour the same main alterations of the tumour, particularly for ctDNA.5,6 These properties prompted us to assess whether these markers could be used as surrogate markers of therapeutic efficacy.
Approximately 80 and 40% of mCRC patients are diagnosed with increased pre-treatment CEA and CA19-9 levels, respectively.7,8,9 However, although baseline values are reported to be prognostic, a definitive cut-off remains lacking, and a single measurement is insufficient to reflect tumour dynamics. The CEA response during the first cycles of treatment may be a surrogate marker of outcome.10,11,12,13 In a retrospective analysis of the randomised FIRE-3 trial, a CEA decrease using a cut-off value at 75% was associated with response to both treatments but was faster and greater in the FOLFIRI–cetuximab arm compared with the FOLFIRI–bevacizumab arm.12 In a series of 122 mCRC patients treated with chemotherapy (CT) alone, we reported that the slope of an exponential-regressive curve connecting the semi-logarithmic values of CEA was predictive of disease progression with a negative predictive value of 95.3%.10
Baseline levels of cfDNA, ctDNA or CTC are also associated with tumour burden and are clinically relevant for predicting prognosis or response in mCRC.14,15,16,17,18,19,20 A few studies recently reported that variations of ctDNA during CT may also be a marker of response to treatment and prognosis.21,22,23 Regarding CTCs, their detection at baseline or during treatment using immunomagnetic or size-based devices is also prognostic in mCRC.14,15,24,25,26 Interestingly, based on a retrospective analysis of available CEA measurements, both CTC and CEA markers contributed to prognosis.27
To our knowledge, all these markers have never been evaluated concomitantly in patients with mCRC. In this context, the aim of the present prospective study was to confirm the clinical value of the CEA kinetics (CEA slope) and to evaluate the predictive and prognostic impact of serial measurements of CA19-9, cfDNA, ctDNA and CTC in a cohort of patients treated for mCRC.
Methods
Study population
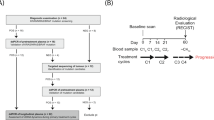
The study was prospectively conducted in five French centres from 11/2010 to 08/2014. The patients were over the age of 18 years, had a histologically proven stage IV colorectal adenocarcinoma with at least one measurable lesion and an Eastern Cooperative Oncology Group (ECOG) performance ≤2. All the patients had a CEA > 5 ng/mL and/or CA19-9 > 30 U/mL and started a CT-based regimen as first or subsequent lines with a free-interval period from the last cycle of at least 2 weeks. All common baseline characteristics and outcomes were collected. The evaluation of the response to treatment was assessed by investigators at baseline and every 12 weeks according to RECIST criteria (version 1.1).3 For each patient, blood samples were prospectively collected into a single EDTA tube (4 mL) for CEA and CA19-9 and 2 EDTA tubes (4 mL) for circulating DNA (cDNA) and CTC. Blood samples were obtained at each cycle from baseline (T0) to the fourth cycle for CEA and CA19-9 (4 sampling times) and at T0 and 6 weeks (W6, i.e. third or fourth cycle) (2 sampling times) for cDNA and CTC detection. Paraffin-embedded tissues were retrospectively collected from each participating centre for KRAS, NRAS, and BRAF mutational status determination. The study was approved by the institutional review board (Northwest I), and all the patients provided written informed consent (NCT01212510). The study was performed in accordance with the Declaration of Helsinki. All the authors had access to the study data and reviewed and approved the final manuscript.
Isolation and quantification of cDNA
All the samples were processed within 2 h after collection with centrifugation at 2700 × g for 20 min and were stored at −80 °C. DNA was extracted from 1 to 2 mL of plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Antwerp, Belgium) according to the manufacturer’s instructions and eluted in 30 µL of AVE buffer. Total cfDNA quantification was performed by a fluorimetric method, as previously described,28 and these data are expressed as ng/mL of plasma.
ctDNA analysis
The ctDNA analysis was centralised at INSERM U1245 unit (Rouen University Hospital), and detection was based on the results of the KRAS, NRAS, and BRAF mutational status in the corresponding tumour tissue. A chip-based dPCR platform (QuantStudio TM 3D Digital PCR System; Life Technologies, Carlsbad, CA) was used for mutation detection in the ctDNA as previously described.28,29 As ctDNA analysis was performed using dPCR and simplex assay in plasma samples, it was necessary to know the type of RAS or BRAF mutations identified in tumour tissue. So, ctDNA analysis was not performed for patients without RAS or BRAF mutations in tumour tissue. The details of the ctDNA analysis are provided in Supplementary Material and Supplementary Tables 1–3.
CTC analysis
After collection, the blood samples were maintained at room temperature, processed within 3 h and analysed using the size-based Screencell® enrichment system as previously described.30 After haematoxylin–eosin–safran staining, enumeration and analysis of the structural characterisation of the CTCs were performed by an experienced cytopathologist in one of the three centres participating in this study (Department of Pathology (Rouen University Hospital, Caen University Hospital and Centre François Baclesse (Caen))). CTCs were classified as positive or negative based on cytomorphological features. The cells were considered positive (i.e. CTCs) based on the presence of at least two of the following criteria: nuclear diameter >14 μm, anisonucleosis (ratio > 0.5), irregular nuclei, high nucleo-cytoplasmic ratio, or the presence of three-dimensional sheets. Cells were considered negative (i.e. circulating non-tumour cells) based on the absence of nuclear or cytological atypies.
CEA and CA19-9 analysis
CEA and CA19-9 analyses were centralised at the Institut de Biologie Clinique (Rouen University Hospital) and Department of Biopathology (Centre Henri Becquerel (Rouen)), respectively. They were performed on a Cobas 8000e602 analyser (Hitachi High Technologies Corp., Tokyo, Japan) using an electrochemiluminescence immunoassay method and on a Kryptor (B.R.A.H.M.S., Hennigsdorf, Germany) using a fluorescence immunoassay method. The laboratories responsible for the analytic methods were taking part in appropriate and relevant external quality assurance schemes. The serial measurements for each patient were analysed in the same run to ensure optimal accuracy. The following tumour marker levels were considered normal: ≤5 ng/mL for CEA and ≤30 UI/mL for CA19-9.
Statistical analysis
The primary endpoint was the validation of the CEA slope for the prediction of progressive disease (PD) at 12 weeks using the 0.05 threshold, which was previously reported.10 The secondary endpoints were PD and progression-free survival (PFS) and overall survival (OS) analyses according to the baseline values and kinetics of the circulating markers (CEA, CA19-9, cDNA and CTCs). A sample of 44 patients with PD at 12 weeks was calculated to validate CEA kinetics with a confidence interval (CI) +/−15%. Considering that PD occurred in approximately 35% of the patients who received first-line treatment with doublet CT and a biologic agent, a sample size of at least 130 patients with available CEA kinetics was determined. Finally, a sample size of 200 patients was planned by considering patients who were lost to follow-up and patients with a CEA ≤ 5 ng/mL at baseline or with <3 samples at 12 weeks of evaluation. The sensitivity (Se), specificity (Sp), the likelihood positive (L+) and negative (L−) ratio, the positive predictive value (PPV), the negative predictive value (NPV), and the diagnostic accuracy odds ratio (DOR L+/L−) were calculated for CEA kinetics. The biomarker kinetics threshold was determined by dichotomising by the median value. Every biomarker was analysed in a different model with a different prognosis score. The PFS was defined as the time from inclusion to disease progression or death from any cause, whichever occurred first. The OS was defined as the time from inclusion to death from any cause. The data cut-off date was May 03, 2020. The survival curves were calculated using the Kaplan–Meier method and were compared with log-rank test. Logistic regression models were used for tumour response at 12 weeks. Proportional hazards and Cox models were used for OS and PFS. Adjustments of the clinical and histological baseline findings were performed by a prognosis score31 that included the following variables: ECOG performance status, tumour location, surgery of the primary tumour, synchronous/metachronous metastasis, number of metastasis sites, CT line, use of targeted therapies, and mutational status (RAS/BRAF mutation or no mutation). The prognosis score was computed for the group that had no or a low level of expression to the biomarker (control group). For analysis of each biomarker, observations with missing data were excluded. Correlations between the baseline clinical variables and the biomarker values were assessed using Mann–Whitney (two groups) and Kruskal–Wallis tests (three groups). Significance level was 0.05 for all the tests. No adjustment for multiple tests was performed. Data were analysed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Patients and sample characteristics
A total of 192 patients were included, and their characteristics are listed in Table 1. A total of 134 (69.8%) patients were included in the first-line CT. The regimens included CT plus biologic agents in 155 (80.7%) patients, CT alone in 31 (16.2%) and anti-epidermal growth factor receptor monotherapy in 6 (3.1%) (Supplementary Table 4). RAS and BRAF mutations were identified in tumour tissues of 90 (46.9%) and 11 (5.7%) patients, respectively. The overall median PFS and OS were 6.5 (95% CI: 5.8–7.4) months and 15.8 (95% CI: 14.7–18.8) months for the whole population, 7 (95% CI: 6.0–9.0) months and 18.4 (95% CI: 14.8–21.3) months for patients without RAS and BRAF mutations, 6.3 (95% CI: 5.1–7.4) months and 15.3 (95% CI: 12.9–18.8) months for patients with RAS mutations and 2.8 (95% CI: 1.3–7.5) months and 8.1 (95% CI: 2.9–14.9) months for patients with BRAF mutations, respectively.
Circulating marker characteristics
The number of samples for each marker and for which the baseline and kinetics value analysis were available are indicated in Fig. 1. The median baseline values of CEA, CA19-9, cfDNA and ctDNA were 97 ng/mL, 89 UI/mL, 24.4 ng/mL and 14.3%, respectively. ctDNA was detectable at baseline in 82 (92.1%) of the patients with an RAS or BRAF mutation identified in tumour tissue and blood samples available (n = 89). More details about ctDNA detection rate according to specific mutation are indicated in Supplementary Data.
*Patients for which the results of circulating markers and 12-week evaluation were available. CA19-9 carbohydrate antigen 19-9, CEA carcinoembryonic antigen, cfDNA cell-free DNA, CTC circulating tumour cells, ctDNA circulating tumour DNA, WT wild type. †Kinetics were estimated for living non-progressive patients having a positive baseline value and two or three further measures at fourth cycle. ‡Only CTC baseline-positive patients underwent kinetic analysis.
Correlations between baseline biomarker values and patient characteristics
Significant correlations were noted between the CEA pre-treatment values and CA19-9 (p = 0.0149), cfDNA (p < 0.0001) and ctDNA (p < 0.0001). Baseline cfDNA was also significantly correlated with CA19-9 (p < 0.0001) and ctDNA (p < 0.0001). As shown in Supplementary Table 5, baseline values of circulating markers were associated with several patient characteristics.
Primary objective of CEA kinetics and outcomes
A CEA kinetic slope >0.05 was observed in 25/159 (15.7%) of the patients. PD was significantly associated with a CEA slope >0.05 and was observed in 19/25 (76%) of the patients with a high CEA slope versus in 22/134 (14.4%) of the patients with a low CEA slope (p < 0.0001; Fig. 2a). This corresponded to a Se of 46% (95% CI: 31–63), a Sp of 95% (95% CI: 89–98), a PPV of 76% (95% CI: 55–91), a NPV of 84% (95% CI: 76–89) and a DOR of 16.1 (Fig. 2a). In univariate analysis, CEA kinetics were significantly associated with PFS and OS (Fig. 3a, c). In multivariate analysis, CEA kinetics were still significantly associated with PD (odds ratio adjusted (ORa) = 16.79; 95% CI: 5.94–47.44, p < 0.0001), PFS (hazard ratio adjusted (HRa) = 3.66; 95% CI: 2.29–5.85, p < 0.0001) and OS (HRa = 2.42; 95% CI: 1.53–3.81, p = 0.0001) (Fig. 4a, b). The rate of patients with CEA kinetic slope >0.05 according to type of treatment is indicated in Supplementary Table 1. For the two main subgroups (i.e. on the one hand, FOLFOX or FOLFIRI (subgroup 1, n = 48) and on the other hand, FOLFOX or FOLFIRI + bevacizumab (subgroup 2, n = 67)), the threshold remained relevant and was significantly associated with PD (subgroup 1, OR = 12.07; 95% CI: 2.45–59.54, p = 0.0022 and subgroup 2, OR = 17.17; 95% CI: 2.82–104.58, p = 0.002) as well as decreased PFS (subgroup 1, HRa = 2.43; 95% CI: 1.13–5.24, p = 0.024 and subgroup 2, HRa = 2.64; 95% CI: 1.1–6.32, p = 0.029) in multivariate analysis.
Prediction of progression according to (a) 0.05 threshold CEA kinetics value previously identified (primary endpoint), (b) baseline circulating marker median value and (c) kinetics circulating marker median value. CA19-9, carbohydrate antigen 19-9; CEA, Carcinoembryonic antigen; cfDNA, cell-free DNA; CTC, circulating tumour cells; ctDNA, circulating tumour DNA; L-, likelihood negative ratio; L+, likelihood positive ratio; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value; Se, sensitivity; Sp, specificity.
Kaplan–Meier curves of a progression-free survival (PFS) and c overall survival (OS) by the CEA kinetics dichotomised at the previously identified threshold (i.e. 0.05). Kaplan–Meier curves of b PFS and d OS by the circulating scoring system (CSS) dichotomised at the 2 value (i.e. <2 and ≥2). B-spline estimates of the hazard ratio for the CEA kinetics (baseline adjusted) (e) and CSS (f). Progression-free survival and overall survival spline curves are represented by the blue and yellow lines, respectively. CEA carcinoembryonic antigen, HR hazard ratio, PFS progression-free survival, OS overall survival.
Forest plot of hazard ratios for (a) progression-free survival and (b) overall survival. The hazard ratio (HR) of each variable was calculated separately and was adjusted for clinical and histological covariates using a prognosis score of well-known prognostic variables, i.e., WHO performance status, tumour location, surgery of the primary tumour, synchronous/metachronous metastasis, number of metastases sites, chemotherapy line, use of targeted therapies, and mutational status (KRAS/NRAS/BRAF mutations or no mutation). CA 19-9, carbohydrate antigen 19-9; CEA, Carcinoembryonic antigen; cfDNA, cell-free DNA; CI, confidence interval; ctDNA, circulating tumour DNA; CTC, circulating tumour cells; HR, hazard ratio; No, total number of patients; PFS, progression-free survival; OS, overall survival.
CEA, CA19-9, cfDNA, ctDNA and CTC baseline values and outcomes
By dichotomising by the median value, cfDNA, ctDNA and CA19-9 were associated with PD (Fig. 2b), PFS and OS in the univariate analysis. In the multivariate analysis, cfDNA and ctDNA were both significantly associated with PD (ORa = 2.41; 95% CI: 1.26–4.64, p = 0.0082 and ORa = 3.37; 95% CI: 1.34–8.5, p = 0.0099, respectively), PFS (HRa = 1.87; 95% CI: 1.36–2.56, p = 0.0001 and HRa = 1.74; 95% CI: 1.13–2.69, p = 0.0127, respectively) and OS (HRa = 2.28; 95% CI: 1.65–3.16, p < 0.0001 and HRa = 2.19; 95% CI: 1.39–3.46, p = 0.0008, respectively) (Fig. 4). The baseline CEA value was not associated with PD (ORa = 0.67; 95% CI: 0.37–1.24, p = 0.20) but was significantly associated with PFS (HRa = 1.41, 95% CI: 1.05–1.90, p = 0.0228) and OS (HRa = 1.54; 95% CI: 1.14–2.09, p = 0.0053). The baseline CA19-9 value was significantly associated with PD (ORa = 2.43; 95% CI: 1.31–4.51, p = 0.0047) and OS (HRa = 1.96; 95% CI: 1.44–2.67, p < 0.0001) (Fig. 4) but not with PFS (HRa = 1.23, 95% CI: 0.92–1.66, p = 0.16). The baseline CTC value (positive versus negative) was not significantly associated with PD (ORa = 1.35; 95% CI: 0.68–2.70, p = 0.39), PFS (HRa = 0.86; 95% CI: 0.62–1.19, p = 0.36) or OS (HRa = 0.86, 95% CI: 0.62–1.20, p = 0.38).
cfDNA, ctDNA and CA19-9 kinetics and outcomes
In univariate analysis, CA19-9 kinetics were associated with PD (Fig. 2c) but not with PFS or OS. In multivariate analysis, the kinetics of CA19-9, cfDNA and ctDNA were associated with PD (ORa = 8.67; 95% CI: 2.99–25.11, p = 0.0001; ORa = 3.6; 95% CI: 1.17–11.12, p = 0.0257 and ORa = 4.81; 95% CI: 1.39–16.69, p = 0.0133, respectively). Regarding the survival outcomes, CA19-9 kinetics were independently associated with PFS (HRa = 1.92; 95% CI: 1.26–2.93, p = 0.0025) and OS (HRa = 2.54; 95% CI: 1.64–3.92, p < 0.0001), and ctDNA kinetics were significantly associated with OS (HRa = 2.33; 95% CI: 1.32–4.09, p = 0.0033) but not with PFS (HRa = 1.60; 95% CI: 0.92–2.79, p = 0.10) (Fig. 4). The cfDNA kinetics were not significantly associated with PFS (HRa = 1.43, 95% CI: 0.93–2.19, p = 0.10) or OS (HRa = 1.37, 95% CI: 0.89–2.13, p = 0.16). CTC kinetics were not significantly associated with PD (ORa = 1.80, 95% CI: 0.66–4.92, p = 0.25), PFS (HRa = 1.06, 95% CI: 0.69–1.63, p = 0.79) or OS (HRa = 1.12, 95% CI: 0.72–1.74, p = 0.62).
Circulating scoring system (CSS)
We performed an exploratory analysis to design a CSS using three markers that were available for the majority of patients and associated with a relevant prognostic value. This score was based on CEA kinetics, CA19-9 and ctDNA/cfDNA baseline values. For each patient, ctDNA baseline value was used if available instead of cfDNA baseline value, whereas cfDNA baseline value was used if ctDNA analysis was not performed due to the lack of mutation identified in tumour tissue. The CSS was comprised of 0 to 3 markers. Each variable was scored 0 or 1 as follows: 0 if CEA kinetic slope ≤0.05 or CA19-9 baseline value <89 UI/mL or ctDNA baseline value <14.3% or cfDNA baseline value <24.4 ng/mL (if ctDNA not analysed) and 1 if CEA kinetic slope >0.05 or CA19-9 baseline value ≥89 UI/mL or ctDNA baseline value ≥14.3% or cfDNA baseline value ≥24.4 ng/mL. Only patients with at least two markers available were included in the CSS analysis. The CSS score was applicable in 171/192 (89%) patients who were classified with low risk (CSS < 2; n = 116 (67.8%)) and high risk of PD (CSS ≥2; n = 55 (32.2%). The rate of PD was 17.2% (20/116) and 52.7% (n = 29/55) (p < 0.0001), respectively (Fig. 2c). In the multivariate analysis, this score was still significantly associated with PD (ORa = 5.03; 95% CI: 2.45–10.31, p < 0.0001) and was also associated with PFS (HRa = 1.94; 95% CI: 1.39–2.7, p = 0.0001) and OS (HRa = 2.93; 95% CI: 2.04–4.21, p < 0.0001) (Fig. 4a, b).
Discussion
To our knowledge, the present study is the first to prospectively evaluate the main circulating markers, namely, CEA, CA19-9, cfDNA, ctDNA and CTC, in mCRC patients. The primary endpoint of the study was met, demonstrating that CEA kinetics using three or four measurements was relevant to monitor patients treated for mCRC. Using a threshold of 0.05 for the CEA slope, PD was identified with a PPV and an NPV of 76 and 83%, respectively, and a CEA kinetics value > 0.05 was also identified as an independent marker of PD and lower survival rates. We also showed that other circulating markers, such as CA19-9, cfDNA and ctDNA but not CTC, were also associated with outcome. Interestingly, we found that a CSS based on CEA kinetics, CA19-9 and ctDNA/cfDNA baseline values may also predict response and prognosis. Although the performance of CSS was quite similar with that of CEA kinetics, more patients were identified with a high risk of PD using CSS than CEA slopes (n = 55 with CSS ≥ 2 versus n = 25 with CEA kinetics >0.005, respectively). Furthermore, CSS is applicable to patients with at least two markers available (i.e. CA19-9 and ctDNA/cfDNA baseline values) and is subsequently applicable to patients for whom the ACE value is unknown or less than or equal to the upper limit of normal (approximately 20% mCRC patients). Taken together, all these results highlight that CEA kinetics are a validated prognostic marker and that a composite score integrating CEA, CA19-9 and ctDNA/cfDNA may also provide relevant and added prognostic value. From a clinical point of view, these findings strongly support that interventional studies testing the adaptation of treatment strategies based on circulating marker detection may be of interest in mCRC.
To date, CEA monitoring has not been standardised in mCRC despite its wide use in daily practice. Although pre-treatment levels are associated with tumour burden and prognosis, no consensual cut-off value has been validated to date. Logically, CEA monitoring using several time points appears to be more relevant than only baseline values to monitor the disease under treatment. In a recent exploratory analysis of the FIRE-3 trial comparing first-line FOLFIRI-bevacizumab versus FOLFIRI-cetuximab in KRAS wild-type mCRC, a CEA response was associated with a better radiological response and prognosis in both arms but occurred faster, at greater levels and more frequently with FOLFIRI-cetuximab.12 In contrast to our work, the CEA response was defined by a 75% decrease occurring at any time point versus the baseline value. Moreover, the nadir for the CEA response, which indirectly reflects the maximal effect of treatment, occurred with a delay of approximately 3 months in both arms in that study. From the perspective of further strategies based on circulating marker detection, our results suggested that a CEA kinetic calculation that uses a limited number of time points might also provide earlier relevant information to predict the treatment effect.
The usefulness of the cfDNA and ctDNA analysis is also questionable in mCRC, particularly regarding CEA analysis. As previously reported, we found that cfDNA and ctDNA levels were correlated given that ctDNA corresponds to a part of cfDNA, which reflects the overall DNA released in the bloodstream from normal and tumour cells. For instance, it is suggested that both of these markers may be associated with tumour burden and that the specificity of ctDNA supports its use preferentially for treatment monitoring. Based on the RAS and BRAF genotyping in the primary tumours, we detected the presence of ctDNA in 92.1% of the mutated patients, corresponding to approximately 53% of the entire population. Considering that the sensitivity of the ctDNA detection is positively associated with the number of genes screened, we decided to use only RAS and BRAF because these two alterations are used in daily practice.32,33 Interestingly, our results showed that the median value of ctDNA at baseline was 14.3% with a significant decrease at W6 (i.e. ctDNA slope ≤−0.73 also corresponding to a ≥10-fold or 90% reduction in ctDNA levels) occurring in 37/72 (51.4%) of the patients, and both parameters were associated with disease status at 12 weeks. These results were consistent with those recently reported in 52 mCRC patients with ctDNA detection based on a tumour analysis using a 15-gene panel.21 Indeed, researchers found a ctDNA pre-treatment value of 16.2 ng/mL with a ≥10-fold or 90% reduction in ctDNA levels before cycle 2 of CT in 19/42 (45%) of the patients. In this study, this ≥10-fold or 90% reduction in ctDNA from baseline to before cycle 2 was a better predictor of radiological response, whereas the fold reduction of the CEA levels at this point exhibited no predictive value. Another study reported by Garlan et al. identified a 80% reduction threshold in ctDNA levels from baseline to before cycle 2 or 3, which predicted significantly the objective responding rate (47.1% (ΔctDNA ≥ 80%) versus 0% (ΔctDNA < 80%), p = 0.03).22 Taking into account the larger sample size of the patients, our results showed that the kinetics of each marker, with the exception of CTCs, appeared to be a better predictor of response than the baseline values, and the highest ORa was observed for CEA kinetics. In the new area of liquid biopsies, these findings clearly support that the serial measurements of CEA are also relevant for monitoring mCRC patients and that CEA kinetics should be integrated into future clinical trials concomitantly with other markers, such as CA19-9, cfDNA and ctDNA.
Using a size-based method, we found that approximately two-thirds of patients had detectable CTCs at baseline without a significant clinical impact. These results are conflicting with previous reports and may be partially related with the properties of the method used to detect CTCs.15 Indeed, techniques depending on the expression of epithelial cell markers, such as epithelial cell molecule adhesion molecule (EpCAM), are associated with a proportion of approximately 25% of patients with detectable CTCs compared to 65% in our study. This recognition of epithelial markers may be defective when the well-known mechanism of the epithelial–mesenchymal transition occurs. In contrast, the device used in our work is a filtration-based, marker-independent method that enables CTC detection based on cell size and morphology. Interestingly, the ISET method, which is based on the same physical properties as our technology, was more sensitive than the CellSearch system in detecting CTCs in patients with lung, breast and prostate cancer, with very little mCRC data available.24,34 However, the few studies detecting CTCs using this method have examined a larger set of patients with mCRC than our study. Taken together, CTC detection using the size-based method is feasible in patients with mCRC and appears to be associated with a higher detection rate than standard devices based on EpCAM recognition alone. Although CTC detection using the immunomagnetic separation technique is a prognostic marker for mCRC, breast cancer and prostate cancer, our results suggest that the usefulness of CTC counting with the size-based method does not provide any additional prognostic or predictive value.
There are some limitations in our study. First, only patients with elevated CEA and/or CA19-9 were eligible for this study. Considering that CEA and CA19-9 are increased in approximately 80 and 40% of patients with advanced colorectal cancer, respectively, our results cannot be extrapolated to the remaining non-secreting patients. Second, the ctDNA detection was based on the results of the RAS and BRAF tumour analysis, giving an overall sensitivity of 53% in our population. Despite the fact that cfDNA was successfully analysed in all the patients with a significant correlation with ctDNA, it would also be interesting to include a larger set of genes in the ctDNA analyses to ensure better coverage of patients. However, unlike the NGS-based method, the cost and time required to complete each ctDNA analysis were reasonable and appeared consistent with implementation in daily clinical practice. Thus, using our dPCR-based detection method and a simplex test, the cost per ctDNA analysis was <20 dollars, and the time of analysis was approximately 6 h.
Third, multiple outcomes and biomarkers were tested, leading to an inflated risk of false discovery. However, when we applied a correction for multiple testing procedure using Benjamini–Hochberg procedure with 5% false discoveries, the significance (significant versus nonsignificant) of any of the 30 (5 biomarkers × 2 time points × 3 outcomes) multivariable prognostic tests was not changed (data not shown).
In conclusion, our prospective study confirms that CEA kinetic measurements alone or combined with baseline CA19-9 and ctDNA/cfDNA (CSS) measurements were clinically relevant for the early prediction of the treatment effects and outcome in patients treated for mCRC. With the exception of CTCs, we also found that all other circulating markers, including CA19-9, cfDNA and ctDNA, might play a role in treatment monitoring and prognostic prediction. Taken together, our findings strongly support that interventional studies based on these circulating detection markers are warranted in mCRC patients.
References
Phelip, J. M., Tougeron, D., Léonard, D., Benhaim, L., Desolneux, G., Dupré, A. et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 51, 1357–1363 (2019).
Van Cutsem, E., Cervantes, A., Adam, R., Sobrero, A., Van Krieken, J. H., Aderka, D. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Hanash, S. M., Baik, C. S. & Kallioniemi, O. Emerging molecular biomarkers−blood-based strategies to detect and monitor cancer. Nat. Rev. Clin. Oncol. 8, 142–150 (2011).
Corcoran, R. B. & Chabner, B. A. Application of cell-free DNA analysis to cancer treatment. N. Engl. J. Med. 379, 1754–1765 (2018).
Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 20, 71–88 (2019).
Filella, X., Molina, R., Grau, J. J., Piqué, J. M., Garcia-Valdecasas, J. C., Astudillo, E. et al. Prognostic value of CA 19.9 levels in colorectal cancer. Ann. Surg. 216, 55–59 (1992).
Hanke, B., Riedel, C., Lampert, S., Happich, K., Martus, P., Parsch, H. et al. CEA and CA 19-9 measurement as a monitoring parameter in metastatic colorectal cancer (CRC) under palliative first-line chemotherapy with weekly 24-hour infusion of high-dose 5-fluorouracil (5-FU) and folinic acid (FA). Ann. Oncol. 12, 221–226 (2001).
Duffy, M. J., van Dalen, A., Haglund, C., Hansson, L., Klapdor, R., Lamerz, R. et al. Clinical utility of biochemical markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines. Eur. J. Cancer 39, 718–727 (2003).
Iwanicki-Caron, I., Di Fiore, F., Roque, I., Astruc, E., Stetiu, M., Duclos, A. et al. Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J. Clin. Oncol. 26, 3681–3686 (2008).
Petrioli, R., Licchetta, A., Roviello, G., Pascucci, A., Francini, E., Bargagli, G. et al. CEA and CA19.9 as early predictors of progression in advanced/metastatic colorectal cancer patients receiving oxaliplatin-based chemotherapy and bevacizumab. Cancer Invest. 30, 65–71 (2012).
Michl, M., Stintzing, S., Fischer von Weikersthal, L., Decker, T., Kiani, A., Vehling-Kaiser, U. et al. CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial). Ann. Oncol. 27, 1565–1572 (2016).
Jia, J., Zhang, P., Gou, M., Yang, F., Qian, N., Dai, G. The role of serum CEA and CA19-9 in efficacy evaluations and progression-free survival predictions for patients treated with cetuximab combined with FOLFOX4 or FOLFIRI as a first-line treatment for advanced colorectal cancer. Dis Markers 2019, 6812045 (2019).
Cohen, S. J., Punt, C. J. A., Iannotti, N., Saidman, B. H., Sabbath, K. D., Gabrail, N. Y. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Tol, J., Koopman, M., Miller, M. C., Tibbe, A., Cats, A., Creemers, G. J. M. et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann. Oncol. 21, 1006–1012 (2010).
Spindler, K. L. G., Pallisgaard, N., Vogelius, I. & Jakobsen, A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin. Cancer Res. 18, 1177–1185 (2012).
Spindler, K. L. G., Appelt, A. L., Pallisgaard, N., Andersen, R. F., Brandslund, I. & Jakobsen, A. Cell-free DNA in healthy individuals, noncancerous disease and strong prognostic value in colorectal cancer. Int. J. Cancer 135, 2984–2991 (2014).
Tabernero, J., Lenz, H. J., Siena, S., Sobrero, A., Falcone, A., Ychou, M. et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 16, 937–948 (2015).
El Messaoudi, S., Mouliere, F., Du Manoir, S., Bascoul-Mollevi, C., Gillet, B., Nouaille, M. et al. Circulating DNA as a strong multi-marker prognostic tool for metastatic colorectal cancer patient management care. Clin. Cancer Res. 22, 3067–3077 (2016).
Hamfjord, J., Guren, T. K., Dajani, O., Johansen, J. S., Glimelius, B., Sorbye, H. et al. Total circulating cell-free DNA as a prognostic biomarker in metastatic colorectal cancer before first-line oxaliplatin-based chemotherapy. Ann. Oncol. 30, 1088–1095 (2019).
Tie, J., Kinde, I., Wang, Y., Wong, H. L., Roebert, J., Christie, M. et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 26, 1715–1722 (2015).
Garlan, F., Laurent-Puig, P., Sefrioui, D., Siauve, N., Didelot, A., Sarafan-Vasseur, N. et al. Early evaluation of circulating tumor DNA as marker of therapeutic efficacy in metastatic colorectal cancer patients (PLACOL Study). Clin. Cancer Res. 23, 5416–5425 (2017).
Parikh, A. R., Mojtahed, A., Schneider, J. L., Kanter, K., Van Seventer, E. E., Fetter, I. J. et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin. Cancer Res. 26, 1877–1885 (2020).
Souza, E., Silva, V., Chinen, L. T. D., Abdallah, E. A., Damascena, A., Paludo, J., Chojniak, R. et al. Early detection of poor outcome in patients with metastatic colorectal cancer: tumor kinetics evaluated by circulating tumor cells. Onco Targets Ther. 9, 7503–7513 (2016).
Ma, B., King, A. D., Leung, L., Wang, K., Poon, A., Ho, W. M. et al. Identifying an early indicator of drug efficacy in patients with metastatic colorectal cancer-a prospective evaluation of circulating tumor cells, 18F-fluorodeoxyglucose positron-emission tomography and the RECIST criteria. Ann. Oncol. 28, 1576–1581 (2017).
Seeberg, L. T., Brunborg, C., Waage, A., Hugenschmidt, H., Renolen, A., Stav, I. et al. Survival impact of primary tumor lymph node status and circulating tumor cells in patients with colorectal liver metastases. Ann. Surg. Oncol. 24, 2113–2121 (2017).
Aggarwal, C., Meropol, N. J., Punt, C. J., Iannotti, N., Saidman, B. H., Sabbath, K. D. et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann. Oncol. 24, 420–428 (2013).
Sefrioui, D., Sarafan-Vasseur, N., Beaussire, L., Baretti, M., Gangloff, A., Blanchard, F. et al. Clinical value of chip-based digital-PCR platform for the detection of circulating DNA in metastatic colorectal cancer. Dig. Liver Dis. 47, 884–890 (2015).
Sefrioui, D., Mauger, F., Leclere, L., Beaussire, L., Di Fiore, F., Deleuze, J. F. et al. Comparison of the quantification of KRAS mutations by digital PCR and E-ice-COLD-PCR in circulating-cell-free DNA from metastatic colorectal cancer patients. Clin. Chim. Acta 465, 1–4 (2016).
Iwanicki-Caron, I., Basile, P., Toure, E., Antonietti, M., Lecleire, S., Di Fiore, A. et al. Usefulness of circulating tumor cell detection in pancreatic adenocarcinoma diagnosis. Am. J. Gastroenterol. 108, 152–155 (2013).
Hansen, B. B. The prognostic analogue of the propensity score. Biometrika 95, 481–488 (2008).
Thierry, A. R., El Messaoudi, S., Mollevi, C., Raoul, J. L., Guimbaud, R., Pezet, D. et al. Clinical utility of circulating DNA analysis for rapid detection of actionable mutations to select metastatic colorectal patients for anti-EGFR treatment. Ann. Oncol. 28, 2149–2159 (2017).
Thierry, A. R., Pastor, B., Jiang, Z. Q., Katsiampoura, A. D., Parseghian, C., Loree, J. M. et al. Circulating DNA demonstrates convergent evolution and common resistance mechanisms during treatment of colorectal cancer. Clin. Cancer Res. 23, 4578–4591 (2017).
Farace, F., Massard, C., Vimond, N., Drusch, F., Jacques, N., Billiot, F. et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 105, 847–853 (2011).
Acknowledgements
The authors thank the patients and their families, investigators and staff from all the participating sites. The authors also thank American Journal Expert (www.aje.com) for English language editing.
Author information
Authors and Affiliations
Contributions
Conception and design: P.M. and F.D.F. Administrative support: P.M. and F.D.F. Provision of study materials or patients: D.S., A.G., M.H., C.E., A.-L.B., A.P., P.G., K.B.-L., M.-P.G., P.M., and F.D.F. Collection and assembly of data: D.S., L.B., A.G., E.T., C.B., A.P., F.Z., F.B., C.T., F.C., J.-C.S., T.F., J.B., N.S.-V., P.M., and F.D.F. Data analysis and interpretation: D.S., L.B., A.G., E.T., C.B., A.P., F.Z., F.B., C.T., J.B., N.S.-V., P.M., and F.D.F. Manuscript writing, final approval of manuscript and accountable for all aspects of the work: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review board (Northwest I), and all patients provided written informed consent (NCT01212510). The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
N/A.
Data availability
Raw data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Funding
This study was supported in part by the Grant “Pierre DURAND & Marie-Thérèse CHEVALIER (2013)” and the pharmaceutical industrial partners Roche, Merck and Amgen France. A grant was also provided by the Charles Nicolle Foundation and the association “des tulipes contre le cancer-Lions Club de France” for financing and acquisition of the digital PCR system used for the detection of circulating tumour DNA. The Northwest Data Center (CTD-CNO) that managed the data was supported by grants from the French National League Against Cancer (LNC) and the French National Cancer Institute (INCa).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Sefrioui, D., Beaussire, L., Gillibert, A. et al. CEA, CA19-9, circulating DNA and circulating tumour cell kinetics in patients treated for metastatic colorectal cancer (mCRC). Br J Cancer 125, 725–733 (2021). https://doi.org/10.1038/s41416-021-01431-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01431-9