Abstract
Background
In the Women’s Health Initiative (WHI) dietary modification (DM) randomised trial, the low-fat dietary intervention reduced deaths from breast cancer (P = 0.02). Extending these findings, secondary analysis examined dietary intervention influence on breast cancer mortality by metabolic syndrome (MS) components.
Methods
In total, 48,835 postmenopausal women with no prior breast cancer were randomised to a low-fat dietary intervention or comparison groups. Four MS components were determined at entry in 45,833 participants: (1) high waist circumference, (2) high blood pressure, (3) high cholesterol and (4) diabetes history. Forest plots of hazard ratios (HRs) were generated with P-values for interaction between randomisation groups and MS component score. Primary outcome was death from breast cancer by metabolic syndrome score.
Results
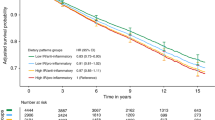
HRs and 95% confidence intervals (CI) for dietary intervention influence on death from breast cancer were with no MS components (n = 10,639), HR 1.09, 95% CI 0.63–1.87; with 1–2 MS components (n = 30,948), HR 0.80, 95% CI 0.62–1.02; with 3–4 MS components (n = 4,246), HR 0.31, 95% CI 0.14–0.69 (interaction P = 0.01).
Conclusions
While postmenopausal women with 3–4 MS components were at higher risk of death from breast cancer, those randomised to a low-fat dietary intervention more likely had reduction in this risk.
Registry
ClinicalTrials.gov (NCT00000611).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Prentice, R. L., Caan, B., Chlebowski, R. T., Patterson, R., Kuller, L. H., Ockene, J. K. et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 295, 629–642 (2006).
Chlebowski, R. T., Aragaki, A. K., Anderson, G. L., Thomson, C. A., Manson, J. E., Simon, M. S. et al. Low-fat dietary pattern and breast cancer mortality in the Women’s Health Initiative randomized controlled trial. J. Clin. Oncol. 35, 2919–2926 (2017).
Chlebowski, R. T., Aragaki, A. K., Anderson, G. L., Pan, K., Neuhouser, M. L., Manson, J. E. et al. Dietary modification and breast cancer mortality: long-term follow-up of the Women’s Health Initiative randomized trial. J. Clin. Oncol. 38, 1419–1428 (2020).
Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
Esposito, K., Chiodini, P., Capuano, A., Bellastella, G., Maiorino, M. I., Rafaniello, C. et al. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause 20, 1301–1309 (2013).
Bhandari, R., Kelley, G. A., Hartley, T. A. & Rockett, I. R. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int. J. Breast Cancer 2014, 189384 (2014).
Lohmann, A. E., Ennis, M., Taylor, S. K. & Goodwin, P. J. Metabolic factors, anthropometric measures, diet, and physical activity in long-term breast cancer survivors: change from diagnosis and comparison to non-breast cancer controls. Breast Cancer Res. Treat. 164, 451–460 (2017).
Dieli-Conwright, C. M., Wong, L., Waliany, S., Bernstein, L., Salehian, B. & Mortimer, J. E. An observational study to examine changes in metabolic syndrome components in patients with breast cancer receiving neoadjuvant or adjuvant chemotherapy. Cancer 122, 2646–2653 (2016).
Bjorge, T., Lukanova, A., Jonsson, H., Tretli, S., Ulmer, H., Manjer, J. et al. Metabolic syndrome and breast cancer in the me-can (metabolic syndrome and cancer) project. Cancer Epidemiol. Biomark. Prev. 19, 1737–1745 (2010).
Gathirua-Mwangi, W. G., Song, Y., Monahan, P. O., Champion, V. L. & Zollinger, T. W. Associations of metabolic syndrome and C-reactive protein with mortality from total cancer, obesity-linked cancers and breast cancer among women in NHANES III. Int. J. Cancer 143, 535–542 (2018).
Simon, M. S., Beebe-Dimmer, J. L., Hastert, T. A., Manson, J. E., Cespedes Feliciano, E. M., Neuhouser, M. L. et al. Cardiometabolic risk factors and survival after breast cancer in the Women’s Health Initiative. Cancer 124, 1798–1807 (2018).
Neuhouser, M. L., Howard, B., Lu, J., Tinker, L. F., Van Horn, L., Caan, B. et al. A low-fat dietary pattern and risk of metabolic syndrome in postmenopausal women: the Women’s Health Initiative. Metab.: Clin. Exp. 61, 1572–1581 (2012).
Chlebowski, R. T., Aragaki, A. K., Anderson, G. L., Simon, M. S., Manson, J. E., Neuhouser, M. L. et al. Association of low-fat dietary pattern with breast cancer overall survival: a secondary analysis of the Women’s Health Initiative randomized clinical trial. JAMA Oncol. 4, e181212 (2018).
Stampfer, M. J., Willett, W. C., Speizer, F. E., Dysert, D. C., Lipnick, R., Rosner, B. et al. Test of the National Death Index. Am. J. Epidemiol. 119, 837–839 (1984).
Jackson, J. M., DeFor, T. A., Crain, A. L., Kerby, T. J., Strayer, L. S., Lewis, C. E. et al. Validity of diabetes self-reports in the Women’s Health Initiative. Menopause 21, 861–868 (2014).
Visvanathan, K., Fabian, C. J., Bantug, E., Brewster, A. M., Davidson, N. E., DeCensi, A. et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J. Clin. Oncol. 37, 3152–3165 (2019).
Fisher, B., Costantino, J. P., Wickerham, D. L., Cecchini, R. S., Cronin, W. M., Robidoux, A. et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 97, 1652–1662 (2005).
Cuzick, J., Sestak, I., Cawthorn, S., Hamed, H., Holli, K., Howell, A. et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 16, 67–75 (2015).
Chlebowski, R. T. IBIS-I tamoxifen update: maturity brings questions. Lancet Oncol. 16, 7–9 (2015).
Goss, P. E., Ingle, J. N., Ales-Martinez, J. E., Cheung, A. M., Chlebowski, R. T., Wactawski-Wende, J. et al. Exemestane for breast-cancer prevention in postmenopausal women. N. Engl. J. Med. 364, 2381–2391 (2011).
Cuzick, J., Sestak, I., Forbes, J. F., Dowsett, M., Cawthorn, S., Mansel, R. E. et al. Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial. Lancet 395, 117–122 (2020).
Cuzick, J., Wang, D. Y. & Bulbrook, R. D. The prevention of breast cancer. Lancet 1, 83–86 (1986).
Purdie, C. A., Quinlan, P., Jordan, L. B., Ashfield, A., Ogston, S., Dewar, J. A. et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br. J. Cancer 110, 565–572 (2014).
Dauphine C., Moazzez A., Neal J. C., Chlebowski R. T. & Ozao-Choy J. Single hormone receptor-positive breast cancers have distinct characteristics and survival. Ann. Surg. Oncol. 27, 4687–4694 (2020).
Li, P., Wang, T., Zeng, C., Yang, M., Li, G., Han, J. et al. Association between metabolic syndrome and prognosis of breast cancer: a meta-analysis of follow-up studies. Diabetol. Metab. Syndr. 12, 10 (2020).
Prentice, R. L., Aragaki, A. K., Howard, B. V., Chlebowski, R. T., Thomson, C. A., Van Horn, L. et al. Low-fat dietary pattern among postmenopausal women influences long-term cancer, cardiovascular disease, and diabetes outcomes. The. J. Nutr. 149, 1565–1574 (2019).
Allison, M. A., Aragaki, A. K., Ray, R. M., Margolis, K. L., Beresford, S. A., Kuller, L. et al. A randomized trial of a low-fat diet intervention on blood pressure and hypertension: tertiary analysis of the WHI dietary modification trial. Am. J. Hypertens. 29, 959–968 (2016).
Van Horn, L., Aragaki, A. K., Howard, B. V., Allison, M. A., Isasi, C. R., Manson, J. E. et al. Eating pattern response to a low-fat diet intervention and cardiovascular outcomes in normotensive women: the Women’s Health Initiative. Curr. Dev. Nutr. 4, nzaa021 (2020).
Siervo, M., Lara, J., Chowdhury, S., Ashor, A., Oggioni, C. & Mathers, J. C. Effects of the dietary approach to stop hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br. J. Nutr. 113, 1–15 (2015).
Pocock, S. & White, I. Trials stopped early: too good to be true? Lancet 353, 943–944 (1999).
Montori, V. M., Devereaux, P. J., Adhikari, N. K., Burns, K. E., Eggert, C. H., Briel, M. et al. Randomized trials stopped early for benefit: a systematic review. JAMA 294, 2203–2209 (2005).
Shirani, F., Salehi-Abargouei, A. & Azadbakht, L. Effects of dietary approaches to stop hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition 29, 939–947 (2013).
Acknowledgements
Prior presentation: Some of the findings were presented on June 1, 2019, at the American Society of Clinical Oncology Annual Meeting. The authors acknowledge the following investigators in the WHI Program: Program Office (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford and Nancy Geller. Clinical Coordinating Center: Fred Hutchinson Cancer Research Center, Seattle, WA, Garnet Anderson, Ross Prentice, Andrea LaCroix and Charles Kooperberg. Investigators and Academic Centers (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA), JoAnn E. Manson (MedStar Health Research Institute/Howard University, Washington, DC), Barbara V. Howard (Stanford Prevention Research Center, Stanford, CA), Marcia L. Stefanick (The Ohio State University, Columbus, OH), Rebecca Jackson (University of Arizona, Tucson/Phoenix, AZ), Cynthia A. Thomson (University at Buffalo, Buffalo, NY), Jean Wactawski-Wende (University of Florida, Gainesville/Jacksonville, FL), Marian Limacher (University of Iowa, Iowa City/Davenport, IA), Robert Wallace (University of Pittsburgh, Pittsburgh, PA), Lewis Kuller (Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center, Torrance, CA), Rowan T. Chlebowski (Wake Forest University School of Medicine, Winston-Salem, NC), Sally Shumaker Women’s Health Initiative Memory Study (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. For a list of all the investigators who have contributed to WHI science, please visit http://www.whi.org/publications/WHI_investigators_longlist.pdf. Additional contributions: we thank the Women’s Health Initiative investigators, staff and the trial participants for their outstanding dedication and commitment.
Author information
Authors and Affiliations
Contributions
Conceived the work that led to the submission: K.P., R.T.C. and J.E. Mortimer. Drafting of the paper: K.P. and R.T.C. Statistical analysis: A.K.A. Acquisition of data: A.K.A., M.L.N., M.S.S., B.C., L.S., J.E. Manson, D.L., T.E.R., K.R., C.K. and R.T.C. Played an important role in interpreting the results: K.P., A.K.A., R.T.C., J.L. and J.E. Manson. Critical revision of the paper for important intellectual content: all authors. Approved the final version: all authors. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The WHI project was reviewed and approved by the Fred Hutchinson Cancer Research Center (Fred Hutch) IRB in accordance with the U.S. Department of Health and Human Services regulations at 45 CFR 46 (approval number: IR# 3467-EXT). Participants provided written informed consent to participate. Additional consent to review medical records was obtained through signed written consent. Fred Hutch has an approved FWA on file with the Office for Human Research Protections (OHRP) under assurance number 0001920. The trial was performed in accordance with the Declaration of Helsinki.
Consent to publish
Not applicable.
Data availability
The following data will be made available beginning 1 July 2022: the identified participant data and data dictionary (information about data sharing for the Women’s Health Initiative can be found at www.WHI.org/researchers/data/Documents/WHI%20Data%20Sharing%20Statement.pdf). For these analyses, data will be publicly available two years after the publication of this paper. The following supporting documents are available: statistical/analytical and informed consent form (https://sp.whi.org/researchers/data/Documents/WHI%20Data%20Sharing%20Statement.pdf).
Competing interests
Rowan T. Chlebowski is a consultant for Novartis, AstraZeneca, Genentech, Merck, Immunomedics, and Puma and received honorarium from Novartis and AstraZeneca. None of the other authors report any competing interests related to this study.
Funding information
The WHI program is supported by the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32 and 44221. HHSN268201600003C, HHSN268201600004C and R25CA203650 also partially supported the development of this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, K., Aragaki, A.K., Neuhouser, M.L. et al. Low-fat dietary pattern and breast cancer mortality by metabolic syndrome components: a secondary analysis of the Women’s Health Initiative (WHI) randomised trial. Br J Cancer 125, 372–379 (2021). https://doi.org/10.1038/s41416-021-01379-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01379-w
This article is cited by
-
Cardiometabolic risk factors, physical activity, and postmenopausal breast cancer mortality: results from the Women’s Health Initiative
BMC Women's Health (2022)
-
Long-term dietary intervention influence on physical activity in the Women’s Health Initiative Dietary Modification randomized trial
Breast Cancer Research and Treatment (2022)