Abstract
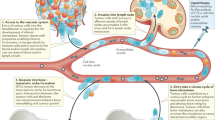
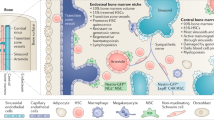
The bone marrow has been widely recognised to host a unique microenvironment that facilitates tumour colonisation. Bone metastasis frequently occurs in the late stages of malignant diseases such as breast, prostate and lung cancers. The biology of bone metastasis is determined by tumour-cell-intrinsic traits as well as their interaction with the microenvironment. The bone marrow is a dynamic organ in which various stages of haematopoiesis, osteogenesis, osteolysis and different kinds of immune response are precisely regulated. These different cellular components constitute specialised tissue microenvironments—niches—that play critical roles in controlling tumour cell colonisation, including initial seeding, dormancy and outgrowth. In this review, we will dissect the dynamic nature of the interactions between tumour cells and bone niches. By targeting certain steps of tumour progression and crosstalk with the bone niches, the development of potential therapeutic approaches for the clinical treatment of bone metastasis might be feasible.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wan, L., Pantel, K. & Kang, Y. Tumor metastasis: moving new biological insights into the clinic. Nat. Med. 19, 1450–1464 (2013).
Fidler, I. J. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Coleman, R. E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 27, 165–176 (2001).
Macedo, F., Ladeira, K., Pinho, F., Saraiva, N., Bonito, N., Pinto, L. et al. Bone metastases: an overview. Oncol. Rev. 11, 321–321 (2017).
Kimura, T. Multidisciplinary approach for bone metastasis: a review. Cancers (Basel) 10, 156 (2018).
Guzik, G. Results of the treatment of bone metastases with modular prosthetic replacement—analysis of 67 patients. J. Orthop. Surg. Res. 11, 20 (2016).
Guise, T. A., Kozlow, W. M., Heras-Herzig, A., Padalecki, S. S., Yin, J. J. & Chirgwin, J. M. Molecular mechanisms of breast cancer metastases to bone. Clin. Breast Cancer 5(Suppl), S46–S53 (2005).
Pan, H., Gray, R., Braybrooke, J., Davies, C., Taylor, C., McGale, P. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Croucher, P. I., McDonald, M. M. & Martin, T. J. Bone metastasis: the importance of the neighbourhood. Nat. Rev. Cancer 16, 373–386 (2016).
Obenauf, A. C. & Massague, J. Surviving at a distance: organ-specific metastasis. Trends Cancer 1, 76–91 (2015).
Ghajar, C. M. Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer 15, 238–247 (2015).
Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 (2007).
Karsenty, G. & Wagner, E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002).
Harada, S. & Rodan, G. A. Control of osteoblast function and regulation of bone mass. Nature 423, 349–355 (2003).
Franz-Odendaal, T. A., Hall, B. K. & Witten, P. E. Buried alive: how osteoblasts become osteocytes. Dev. Dyn. 235, 176–190 (2006).
Frost, H. M. In vivo osteocyte death. J. Bone Joint Surg. Am. 42, 138–143 (1960).
Ikeda, K. & Takeshita, S. The role of osteoclast differentiation and function in skeletal homeostasis. J. Biochem. 159, 1–8 (2016).
Chitu, V. & Stanley, E. R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 (2006).
Gow, D. J., Sester, D. P. & Hume, D. A. CSF-1, IGF-1, and the control of postnatal growth and development. J. Leukoc. Biol. 88, 475–481 (2010).
Haider, M. T., Smit, D. J. & Taipaleenmaki, H. The endosteal niche in breast cancer bone metastasis. Front. Oncol. 10, 335 (2020).
Shen, Y. & Nilsson, S. K. Bone, microenvironment and hematopoiesis. Curr. Opin. Hematol. 19, 250–255 (2012).
Frenette, P. S., Pinho, S., Lucas, D. & Scheiermann, C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 31, 285–316 (2013).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Clevers, H. The cancer stem cell: premises, promises and challenges. Nat. Med. 17, 313–319 (2011).
Hsu, Y. C. & Fuchs, E. A family business: stem cell progeny join the niche to regulate homeostasis. Nat. Rev. Mol. Cell Biol. 13, 103–114 (2012).
Moore, K. A. & Lemischka, I. R. Stem cells and their niches. Science 311, 1880–1885 (2006).
Morrison, S. J. & Spradling, A. C. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611 (2008).
Mendez-Ferrer, S., Michurina, T. V., Ferraro, F., Mazloom, A. R., Macarthur, B. D., Lira, S. A. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Yu, V. W. & Scadden, D. T. Heterogeneity of the bone marrow niche. Curr. Opin. Hematol. 23, 331–338 (2016).
Birbrair, A. & Frenette, P. S. Niche heterogeneity in the bone marrow. Ann. N. Y. Acad. Sci. 1370, 82–96 (2016).
Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care (Engl.) 26, e12740 (2017).
Feng, X. & McDonald, J. M. Disorders of bone remodeling. Annu. Rev. Pathol. 6, 121–145 (2011).
Pinho, S., Lacombe, J., Hanoun, M., Mizoguchi, T., Bruns, I., Kunisaki, Y. et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 210, 1351–1367 (2013).
Greenbaum, A., Hsu, Y. M., Day, R. B., Schuettpelz, L. G., Christopher, M. J., Borgerding, J. N. et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 (2013).
Ding, L., Saunders, T. L., Enikolopov, G. & Morrison, S. J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462 (2012).
Park, D., Spencer, J. A., Koh, B. I., Kobayashi, T., Fujisaki, J., Clemens, T. L. et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 (2012).
Ding, L. & Morrison, S. J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Sacchetti, B., Funari, A., Michienzi, S., Di Cesare, S., Piersanti, S., Saggio, I. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Agarwal, P., Isringhausen, S., Li, H., Paterson, A. J., He, J., Gomariz, A. et al. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell 24, 769–784 e766 (2019).
Asada, N., Kunisaki, Y., Pierce, H., Wang, Z., Fernandez, N. F., Birbrair, A. et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223 (2017).
Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C. et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003).
Chatterjee, S., Behnam Azad, B. & Nimmagadda, S. The intricate role of CXCR4 in cancer. Adv. Cancer Res. 124, 31–82 (2014).
Shiozawa, Y., Havens, A. M., Pienta, K. J. & Taichman, R. S. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia 22, 941–950 (2008).
Chen, F., Lin, X., Xu, P., Zhang, Z., Chen, Y., Wang, C. et al. Nuclear export of smads by RanBP3L regulates bone morphogenetic protein signaling and mesenchymal stem cell differentiation. Mol. Cell Biol. 35, 1700–1711 (2015).
Ambrosi, T. H., Longaker, M. T. & Chan, C. K. F. A revised perspective of skeletal stem cell biology. Front. Cell Dev. Biol. 7, 189 (2019).
Acar, M., Kocherlakota, K. S., Murphy, M. M., Peyer, J. G., Oguro, H., Inra, C. N. et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130 (2015).
Crane, G. M., Jeffery, E. & Morrison, S. J. Adult haematopoietic stem cell niches. Nat. Rev. Immunol. 17, 573–590 (2017).
Asada, N., Takeishi, S. & Frenette, P. S. Complexity of bone marrow hematopoietic stem cell niche. Int. J. Hematol. 106, 45–54 (2017).
Sun, Y. X., Schneider, A., Jung, Y., Wang, J., Dai, J., Wang, J. et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J. Bone Miner. Res. 20, 318–329 (2005).
Nervi, B., Ramirez, P., Rettig, M. P., Uy, G. L., Holt, M. S., Ritchey, J. K. et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 113, 6206–6214 (2009).
Shain, K. H., Yarde, D. N., Meads, M. B., Huang, M., Jove, R., Hazlehurst, L. A. et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 69, 1009–1015 (2009).
Ara, T., Song, L., Shimada, H., Keshelava, N., Russell, H. V., Metelitsa, L. S. et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 69, 329–337 (2009).
Brocke-Heidrich, K., Kretzschmar, A. K., Pfeifer, G., Henze, C., Loffler, D., Koczan, D. et al. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood 103, 242–251 (2004).
Ono, M., Kosaka, N., Tominaga, N., Yoshioka, Y., Takeshita, F., Takahashi, R. U. et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal 7, ra63 (2014).
Ramasamy, S. K. Structure and functions of blood vessels and vascular niches in bone. Stem Cells Int. 2017, 5046953 (2017).
Hendriks, M. & Ramasamy, S. K. Blood vessels and vascular niches in bone development and physiological remodeling. Front. Cell Dev. Biol. 8, 602278–602278 (2020).
Lafage-Proust, M. H., Roche, B., Langer, M., Cleret, D., Vanden Bossche, A., Olivier, T. et al. Assessment of bone vascularization and its role in bone remodeling. Bonekey Rep 4, 662 (2015).
Augustin, H. G. & Koh, G. Y. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science 357, eaal2379 (2017).
Sanger, N., Effenberger, K. E., Riethdorf, S., Van Haasteren, V., Gauwerky, J., Wiegratz, I. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011).
Melchior, S. W., Corey, E., Ellis, W. J., Ross, A. A., Layton, T. J., Oswin, M. M. et al. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin. Cancer Res. 3, 249–256 (1997).
Walz, G., Aruffo, A., Kolanus, W., Bevilacqua, M. & Seed, B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science 250, 1132–1135 (1990).
Gunasinghe, N. P., Wells, A., Thompson, E. W. & Hugo, H. J. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 31, 469–478 (2012).
Esposito, M., Mondal, N., Greco, T. M., Wei, Y., Spadazzi, C., Lin, S. C. et al. Bone vascular niche E-selectin induces mesenchymal-epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 (2019).
Carlson, P., Dasgupta, A., Grzelak, C. A., Kim, J., Barrett, A., Coleman, I. M. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Yagiz, K. & Rittling, S. R. Both cell-surface and secreted CSF-1 expressed by tumor cells metastatic to bone can contribute to osteoclast activation. Exp Cell Res. 315, 2442–2452 (2009).
Weilbaecher, K. N., Guise, T. A. & McCauley, L. K. Cancer to bone: a fatal attraction. Nat. Rev. Cancer 11, 411–425 (2011).
Kang, Y. Dissecting tumor-stromal interactions in breast cancer bone metastasis. Endocrinol. Metab. (Seoul) 31, 206–212 (2016).
Ell, B. & Kang, Y. SnapShot: Bone Metastasis. Cell 151, 690–690 e691 (2012).
Roodman, G. D. Mechanisms of bone metastasis. N. Engl. J. Med. 350, 1655–1664 (2004).
Yin, J. J., Selander, K., Chirgwin, J. M., Dallas, M., Grubbs, B. G., Wieser, R. et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Sethi, N., Dai, X., Winter, C. G. & Kang, Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell 19, 192–205 (2011).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Iotsova, V., Caamano, J., Loy, J., Yang, Y., Lewin, A. & Bravo, R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat. Med. 3, 1285–1289 (1997).
Lacey, D. L., Timms, E., Tan, H. L., Kelley, M. J., Dunstan, C. R., Burgess, T. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Lu, X., Mu, E., Wei, Y., Riethdorf, S., Yang, Q., Yuan, M. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Zheng, H., Bae, Y., Kasimir-Bauer, S., Tang, R., Chen, J., Ren, G. et al. Therapeutic antibody targeting tumor- and osteoblastic niche-derived jagged1 sensitizes bone metastasis to chemotherapy. Cancer Cell 32, 731–747 e736 (2017).
Hume, D. A. & MacDonald, K. P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820 (2012).
Westendorf, J. J., Kahler, R. A. & Schroeder, T. M. Wnt signaling in osteoblasts and bone diseases. Gene 341, 19–39 (2004).
Hall, C. L., Bafico, A., Dai, J., Aaronson, S. A. & Keller, E. T. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 65, 7554–7560 (2005).
Ren, D., Dai, Y., Yang, Q., Zhang, X., Guo, W., Ye, L. et al. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J. Exp. Med. 216, 428–449 (2019).
Zhuang, X., Zhang, H., Li, X., Li, X., Cong, M., Peng, F. et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat. Cell Biol. 19, 1274–1285 (2017).
Wang, H., Yu, C., Gao, X., Welte, T., Muscarella, A. M., Tian, L. et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27, 193–210 (2015).
Wang, H., Tian, L., Liu, J., Goldstein, A., Bado, I., Zhang, W. et al. The osteogenic niche is a calcium reservoir of bone micrometastases and confers unexpected therapeutic vulnerability. Cancer Cell 34, 823–839 e827 (2018).
Devignes, C. S., Aslan, Y., Brenot, A., Devillers, A., Schepers, K., Fabre, S. et al. HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc. Natl Acad. Sci. USA 115, E992–E1001 (2018).
Delgado-Calle, J. & Bellido, T. Osteocytes and skeletal pathophysiology. Curr. Mol. Biol. Rep. 1, 157–167 (2015).
Robling, A. G. & Bonewald, L. F. The Osteocyte: New Insights. Annu. Rev. Physiol 82, 485–506 (2020).
Sottnik, J. L., Dai, J., Zhang, H., Campbell, B. & Keller, E. T. Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 75, 2151–2158 (2015).
Delgado-Calle, J., Anderson, J., Cregor, M. D., Hiasa, M., Chirgwin, J. M., Carlesso, N. et al. Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res. 76, 1089–1100 (2016).
Dierkes, C., Kreisel, M., Schulz, A., Steinmeyer, J., Wolff, J. C. & Fink, L. Catabolic properties of microdissected human endosteal bone lining cells. Calcif. Tissue Int. 84, 146–155 (2009).
Johnson, R. W. & Suva, L. J. Hallmarks of Bone Metastasis. Calcif. Tissue Int. 102, 141–151 (2018).
Feuerer, M., Rocha, M., Bai, L., Umansky, V., Solomayer, E. F., Bastert, G. et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int. J. Cancer 92, 96–105 (2001).
Zhang, K., Kim, S., Cremasco, V., Hirbe, A. C., Collins, L., Piwnica-Worms, D. et al. CD8+ T cells regulate bone tumor burden independent of osteoclast resorption. Cancer Res. 71, 4799–4808 (2011).
Lode, H. N., Xiang, R., Dreier, T., Varki, N. M., Gillies, S. D. & Reisfeld, R. A. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood 91, 1706–1715 (1998).
Bidwell, B. N., Slaney, C. Y., Withana, N. P., Forster, S., Cao, Y., Loi, S. et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat. Med. 18, 1224–1231 (2012).
Colucci, S., Brunetti, G., Rizzi, R., Zonno, A., Mori, G., Colaianni, G. et al. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood 104, 3722–3730 (2004).
Monteiro, A. C., Leal, A. C., Goncalves-Silva, T., Mercadante, A. C., Kestelman, F., Chaves, S. B. et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS ONE 8, e68171 (2013).
Zhao, E., Wang, L., Dai, J., Kryczek, I., Wei, S., Vatan, L. et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 1, 152–161 (2012).
Wyckoff, J. B., Wang, Y., Lin, E. Y., Li, J. F., Goswami, S., Stanley, E. R. et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649–2656 (2007).
Mendoza-Reinoso, V., McCauley, L. K. & Fournier, P. G. J. Contribution of macrophages and T cells in skeletal metastasis. Cancers (Basel) 12, 1014 (2020).
Ma, R. Y., Zhang, H., Li, X. F., Zhang, C. B., Selli, C., Tagliavini, G. et al. Monocyte-derived macrophages promote breast cancer bone metastasis outgrowth. J. Exp. Med. 217, e20191820 (2020).
Zhao, E., Xu, H., Wang, L., Kryczek, I., Wu, K., Hu, Y. et al. Bone marrow and the control of immunity. Cell Mol. Immunol. 9, 11–19 (2012).
Zhuang, J., Zhang, J., Lwin, S. T., Edwards, J. R., Edwards, C. M., Mundy, G. R. et al. Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PLoS ONE 7, e48871 (2012).
Sawant, A., Hensel, J. A., Chanda, D., Harris, B. A., Siegal, G. P., Maheshwari, A. et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J. Immunol. 189, 4258–4265 (2012).
Qian, B., Deng, Y., Im, J. H., Muschel, R. J., Zou, Y., Li, J. et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 4, e6562 (2009).
Saji, H., Koike, M., Yamori, T., Saji, S., Seiki, M., Matsushima, K. et al. Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92, 1085–1091 (2001).
Kitamura, T., Qian, B. Z., Soong, D., Cassetta, L., Noy, R., Sugano, G. et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 212, 1043–1059 (2015).
Silzle, T., Kreutz, M., Dobler, M. A., Brockhoff, G., Knuechel, R. & Kunz-Schughart, L. A. Tumor-associated fibroblasts recruit blood monocytes into tumor tissue. Eur. J. Immunol. 33, 1311–1320 (2003).
Huang, B., Lei, Z., Zhao, J., Gong, W., Liu, J., Chen, Z. et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 252, 86–92 (2007).
Kirk, P. S., Koreckij, T., Nguyen, H. M., Brown, L. G., Snyder, L. A., Vessella, R. L. et al. Inhibition of CCL2 signaling in combination with docetaxel treatment has profound inhibitory effects on prostate cancer growth in bone. Int. J. Mol. Sci. 14, 10483–10496 (2013).
Ell, B., Mercatali, L., Ibrahim, T., Campbell, N., Schwarzenbach, H., Pantel, K. et al. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24, 542–556 (2013).
Manthey, C. L., Johnson, D. L., Illig, C. R., Tuman, R. W., Zhou, Z., Baker, J. F. et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol. Cancer Ther. 8, 3151–3161 (2009).
Sloan, E. K., Priceman, S. J., Cox, B. F., Yu, S., Pimentel, M. A., Tangkanangnukul, V. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010).
Morris, E. V. & Edwards, C. M. The role of bone marrow adipocytes in bone metastasis. J. Bone Oncol. 5, 121–123 (2016).
Lucotti, S. & Muschel, R. J. Platelets and Metastasis: New Implications of an Old Interplay. Front. Oncol. 10, 1350 (2020).
Maroni, P. Megakaryocytes in bone metastasis: protection or progression? Cells 8, 134 (2019).
Jackson, W. 3rd, Sosnoski, D. M., Ohanessian, S. E., Chandler, P., Mobley, A., Meisel, K. D. et al. Role of megakaryocytes in breast cancer metastasis to bone. Cancer Res. 77, 1942–1954 (2017).
Li, X., Koh, A. J., Wang, Z., Soki, F. N., Park, S. I., Pienta, K. J. et al. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner. Res. 26, 125–134 (2011).
Shirai, T., Revenko, A. S., Tibbitts, J., Ngo, A. T. P., Mitrugno, A., Healy, L. D. et al. Hepatic thrombopoietin gene silencing reduces platelet count and breast cancer progression in transgenic MMTV-PyMT mice. Blood Adv. 3, 3080–3091 (2019).
Psaila, B., Lyden, D. & Roberts, I. Megakaryocytes, malignancy and bone marrow vascular niches. J. Thromb. Haemost. 10, 177–188 (2012).
Smith, E. P., Specker, B. & Korach, K. S. Recent experimental and clinical findings in the skeleton associated with loss of estrogen hormone or estrogen receptor activity. J. Steroid Biochem. Mol. Biol. 118, 264–272 (2010).
Morrissey, C., Roudier, M. P., Dowell, A., True, L. D., Ketchanji, M., Welty, C. et al. Effects of androgen deprivation therapy and bisphosphonate treatment on bone in patients with metastatic castration-resistant prostate cancer: results from the University of Washington Rapid Autopsy Series. J Bone Miner. Res. 28, 333–340 (2013).
Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K. et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130, 811–823 (2007).
Ren, G., Esposito, M. & Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. (Berl.) 93, 1203–1212 (2015).
Drake, M. T., Clarke, B. L. & Khosla, S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 83, 1032–1045 (2008).
Early Breast Cancer Trialists’ Collaborative, G. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386, 1353–1361 (2015).
Ottewell, P. D., Wang, N., Brown, H. K., Reeves, K. J., Fowles, C. A., Croucher, P. I. et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin. Cancer Res. 20, 2922–2932 (2014).
Lacey, D. L., Boyle, W. J., Simonet, W. S., Kostenuik, P. J., Dougall, W. C., Sullivan, J. K. et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11, 401–419 (2012).
Fizazi, K., Carducci, M., Smith, M., Damiao, R., Brown, J., Karsh, L. et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011).
Kostenuik, P. J., Nguyen, H. Q., McCabe, J., Warmington, K. S., Kurahara, C., Sun, N. et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res. 24, 182–195 (2009).
Oyajobi, B. O., Anderson, D. M., Traianedes, K., Williams, P. J., Yoneda, T. & Mundy, G. R. Therapeutic efficacy of a soluble receptor activator of nuclear factor kappaB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 61, 2572–2578 (2001).
Bone, H. G., McClung, M. R., Roux, C., Recker, R. R., Eisman, J. A., Verbruggen, N. et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J. Bone Miner. Res. 25, 937–947 (2010).
Lotinun, S., Kiviranta, R., Matsubara, T., Alzate, J. A., Neff, L., Luth, A. et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J. Clin. Invest. 123, 666–681 (2013).
Id Boufker, H., Lagneaux, L., Najar, M., Piccart, M., Ghanem, G., Body, J. J. et al. The Src inhibitor dasatinib accelerates the differentiation of human bone marrow-derived mesenchymal stromal cells into osteoblasts. BMC Cancer 10, 298 (2010).
Vandyke, K., Dewar, A. L., Diamond, P., Fitter, S., Schultz, C. G., Sims, N. A. et al. The tyrosine kinase inhibitor dasatinib dysregulates bone remodeling through inhibition of osteoclasts in vivo. J. Bone Miner. Res. 25, 1759–1770 (2010).
Lee, Y. C., Huang, C. F., Murshed, M., Chu, K., Araujo, J. C., Ye, X. et al. Src family kinase/abl inhibitor dasatinib suppresses proliferation and enhances differentiation of osteoblasts. Oncogene 29, 3196–3207 (2010).
Eckhardt, B. L., Francis, P. A., Parker, B. S. & Anderson, R. L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat. Rev. Drug Discov. 11, 479–497 (2012).
Acknowledgements
We thank the members of our laboratory for helpful discussions. We also apologise to the many investigators whose important studies could not be cited directly here owing to space limitations. Illustrations created with Biorender.com.
Author information
Authors and Affiliations
Contributions
F.C. and Y.H. co-wrote and revised the manuscript. Y.K. co-wrote and revised the manuscript and provided overall guidance.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
Y.K. holds equity interest in KayoThera and Firebrand Therapeutics.
Funding information
The work in the authors’ laboratory is supported by grants from the Brewster Foundation, American Cancer Society, Susan G. Komen Foundation, Breast Cancer Research Foundation, the NIH and the U.S. Department of Defense to Y.K.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, F., Han, Y. & Kang, Y. Bone marrow niches in the regulation of bone metastasis. Br J Cancer 124, 1912–1920 (2021). https://doi.org/10.1038/s41416-021-01329-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01329-6
This article is cited by
-
Tissue-specific Tregs in cancer metastasis: opportunities for precision immunotherapy
Cellular & Molecular Immunology (2022)