Abstract
Background
Ranitidine, a histamine 2 blocker, is the standard of care to prevent hypersensitivity reactions (HSRs) caused by paclitaxel infusion. However, the added value of ranitidine in this premedication regimen is controversial. Therefore, we compared the incidence of HSRs during paclitaxel treatment between a standard regimen including ranitidine and a regimen without ranitidine.
Methods
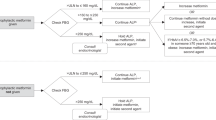
This prospective, pre–post interventional, non-inferiority study compared the standard premedication regimen (N = 183) with dexamethasone, clemastine and ranitidine with a premedication regimen without ranitidine (N = 183). The primary outcome was the incidence of HSR grade ≥3. Non-inferiority was determined by checking whether the upper bound of the two-sided 90% confidence interval (CI) for the difference in HSR rates excluded the +6% non-inferiority margin.
Results
In both the pre-intervention (with ranitidine) and post-intervention (without ranitidine) group 183 patients were included. The incidence of HSR grade ≥3 was 4.4% (N = 8) in the pre-intervention group and 1.6% (N = 3) in the post-intervention group: difference −2.7% (90% CI: −6.2 to 0.1).
Conclusions
As the upper boundary of the 90% CI does not exceed the predefined non-inferiority margin of +6%, it can be concluded that a premedication regimen without ranitidine is non-inferior to a premedication regimen with ranitidine.
Clinical Trial Registration
www.trialregister.nl; NL8173.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Szebeni, J., Muggia, F. M. & Alving, C. R. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: an in vitro study. J. Natl Cancer Inst. 90, 300–306 (1998).
Park, K. H., Pai, J., Song, D. G., Sim, D. W., Park, H. J., Lee, J. H. et al. Ranitidine-induced anaphylaxis: clinical features, cross-reactivity, and skin testing. Clin. Exp. Allergy 46, 631–639 (2016).
Joerger, M. Prevention and handling of acute allergic and infusion reactions in oncology. Ann. Oncol. 23 (Suppl. 10), x313–x319 (2012).
Bristol-Meyers Squibb. Taxol (Paclitaxel) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf (2020).
Choy, H. Taxanes in combined modality therapy for solid tumors. Crit. Rev. Oncol. Hematol. 37, 237–247 (2001).
Vogel, W. H. Infusion reactions: diagnosis, assessment, and management. Clin. J. Oncol. Nurs. 14, E10–E21 (2010).
Common Terminology Criteria for Adverse Events, version 4.0, published 28 May 2009 (v4.03: 14 June 2010) (2020). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx.
Markman, M., Kennedy, A., Webster, K., Kulp, B., Peterson, G., Belinson, J. et al. Paclitaxel-associated hypersensitivity reactions: experience of the gynecologic oncology program of the Cleveland Clinic Cancer Center. J. Clin. Oncol. 18, 102–105 (2000).
Romano, A., Torres, M. J., Castells, M., Sanz, M. L. & Blanca, M. Diagnosis and management of drug hypersensitivity reactions. J. Allergy Clin. Immunol. 127, S67–S73 (2011).
Nokihara, H., Yamamoto, N., Ohe, Y., Hiraoka, M. & Tamura, T. Pharmacokinetics of weekly paclitaxel and feasibility of dexamethasone taper in Japanese patients with advanced non-small cell lung cancer. Clin. Ther. 38, 338–347 (2016).
Bookman, M. A., Kloth, D. D., Kover, P. E., Smolinski, S. & Ozols, R. F. Short-course intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Ann. Oncol. 8, 611–614 (1997).
Weiss, R. B., Donehower, R. C., Wiernik, P. H., Ohnuma, T., Gralla, R. J., Trump, D. L. et al. Hypersensitivity reactions from taxol. J. Clin. Oncol. 8, 1263–1268 (1990).
Markman, M., Kennedy, A., Webster, K., Peterson, G., Kulp, B., Belinson, J. et al. An effective and more convenient drug regimen for prophylaxis against paclitaxel-associated hypersensitivity reactions. J. Cancer Res. Clin. Oncol. 125, 427–429 (1999).
Berger, M. J., Vargo, C., Vincent, M., Shaver, K., Phillips, G., Layman, R. et al. Stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support Care Cancer 23, 2019–2024 (2015).
Yahata, H., Saito, M., Sendo, T., Itoh, Y., Uchida, M., Hirakawa, T. et al. Prophylactic effect of pemirolast, an antiallergic agent, against hypersensitivity reactions to paclitaxel in patients with ovarian cancer. Int. J. Cancer 118, 2636–2638 (2006).
Greenberger, P. A., Patterson, R., Simon, R., Lieberman, P. & Wallace, W. Pretreatment of high-risk patients requiring radiographic contrast media studies. J. Allergy Clin. Immunol. 67, 185–187 (1981).
Wiernik, P. H., Schwartz, E. L., Strauman, J. J., Dutcher, J. P., Lipton, R. B. & Paietta, E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 47, 2486–2493 (1987).
Greenberger, P. A., Patterson, R. & Tapio, C. M. Prophylaxis against repeated radiocontrast media reactions in 857 cases. Adverse experience with cimetidine and safety of beta-adrenergic antagonists. Arch. Intern. Med. 145, 2197–2200 (1985).
Cook, J. & Shuster, S. Lack of effect of H2 blockade in chronic urticaria [proceedings. Br. J. Dermatol. 101 (Suppl. 17), 21–22 (1979).
Greenberger, P., Harris, K. & Patterson, R. The effect of histamine-1 and histamine-2 antagonists on airway responses to histamine in the rhesus monkey. J. Allergy Clin. Immunol. 64, 189–196 (1979).
Demirkan, K., Bozkurt, B., Karakaya, G. & Kalyoncu, A. F. Anaphylactic reaction to drugs commonly used for gastrointestinal system diseases: 3 case reports and review of the literature. J. Investig. Allergol. Clin. Immunol. 16, 203–209 (2006).
Han, T. Y., Jang, W. S., Yu, M., Lee, H. K., Son, S. J., Seo, S. J. et al. Anaphylactic reaction to ranitidine (Zantac(R)). Int. J. Dermatol. 50, 1397–1399 (2011).
HyLown.com. Power and sample size calculator (2020). http://powerandsamplesize.com/Calculators/.
R Core Team (2013). R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
Koppler, H., Heymanns, J. & Weide, R. Dose reduction of steroid premedication for paclitaxel: no increase of hypersensitivity reactions. Onkologie 24, 283–285 (2001).
Sasada, S., Hirashima, T., Nakamura, Y., Takimoto, T., Furukawa, M., Kobayashi, M. et al. Preliminary experience with a modified premedication protocol that included intravenous diphenhydramine and calcium bromide for the prophylaxis of paclitaxel-related hypersensitivity reactions. Int. J. Clin. Oncol. 12, 274–278 (2007).
Hainsworth, J. D. & Greco, F. A. Paclitaxel administered by 1-hour infusion. Preliminary results of a phase I/II trial comparing two schedules. Cancer 74, 1377–1382 (1994).
Aoyama, T., Takano, M., Miyamoto, M., Yoshikawa, T., Soyama, H., Kato, K. et al. Is there any predictor for hypersensitivity reactions in gynecologic cancer patients treated with paclitaxel-based therapy?. Cancer Chemother. Pharmacol. 80, 65–69 (2017).
Acknowledgements
We thank all the nurses from the Department of Medical Oncology from the Erasmus MC Cancer Institute for contributing to the RANISTOP study. This work was earlier presented as a poster on the ESMO Virtual Congress 2020 (1851p).
Author information
Authors and Affiliations
Contributions
R.W.F.v.L. conceived the study. J.M.C., L.v.D., E.O.-d.H., P.M.L.A.v.d.B., R.H.J.M. and R.W.F.v.L. contributed to study design. J.M.C., L.v.D. and E.O.-d.H. had a role in data analysis. J.M.C. and L.v.D. wrote the first draft of the manuscript and contributed to data collection. All authors had a role in the interpretation of the results and critically reviewed the manuscript. J.M.C., L.v.D. and E.O.-d.H. contributed to data preparation and analysis. All authors agreed with the decision to submit for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the medical ethical board of the Erasmus MC, reference number MEC-2018-1499. All included patients gave informed consent. This study was performed in accordance with the Declaration of Helsinki.
Data availability
All data supporting the findings of this study are available within the article and its information files and from the corresponding author upon reasonable request.
Competing interests
R.H.J.M. reports research grants from Astellas, Bayer, Boehringer-Ingelheim, Cristal Therapeutics, Pfizer, Prostakan, Roche and Pamgene. Personal fees from Novartis and Servier; all outside the submitted work. R.W.F.v.L. reports research grants from Astellas, Bayer, Boehringer-Ingelheim, Bristol Myers Squibb, Servier and Roche. Personal fees from Roche, Pfizer and Sanofi; all outside the submitted work. All other authors declare no potential competing interests.
Funding information
This study was funded by the Foundation Mitialto in the Netherlands. Foundation Mitialto had no role in any aspect of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cox, J.M., van Doorn, L., Malmberg, R. et al. The added value of H2 antagonists in premedication regimens during paclitaxel treatment. Br J Cancer 124, 1647–1652 (2021). https://doi.org/10.1038/s41416-021-01313-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01313-0
This article is cited by
-
Drug Utilisation Patterns of Alternatives to Ranitidine-Containing Medicines in Patients Treated with Ranitidine: A Network Analysis of Data from Six European National Databases
Drug Safety (2023)
-
Premedication Protocols to Prevent Hypersensitivity Reactions to Chemotherapy: a Literature Review
Clinical Reviews in Allergy & Immunology (2022)
-
Pre-medication protocols for the prevention of paclitaxel-induced infusion related reactions: a systematic review and meta-analysis
Supportive Care in Cancer (2022)
-
No need for H2-antagonists in premedication regimens for paclitaxel infusions: less is more
British Journal of Cancer (2021)
-
Paclitaxel/ranitidine
Reactions Weekly (2021)