Abstract
Tumour budding in colorectal cancer has become an important prognostic factor. Represented by single cells or small tumour cell clusters at the invasion front of the tumour mass, these tumour buds seem to reflect cells in a ‘hybrid’ state of epithelial–mesenchymal transition, and evidence indicates that the presence of these entities is associated with lymph node metastasis, local recurrence and distant metastatic disease. The International Tumour Budding Consensus Conference (ITBCC) has highlighted a scoring system for the reporting of tumour budding in colorectal cancer, as well as different clinical scenarios that could affect patient management. Other organs are not spared: tumour budding has been described in numerous gastrointestinal and non-gastrointestinal cancers. Here, we give an update on ITBCC validation studies in the context of colorectal cancer and the clinical implications of tumour budding throughout the upper gastrointestinal and pancreatico-biliary tract.
Similar content being viewed by others
Background
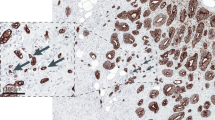
Interest in tumour budding and its clinical implications has surged over the past few years. Tumour budding is defined by the presence of single tumour cells or small clusters of cells within the tumour centre (‘intratumoural’ budding, ITB) (Fig. 1a) or at the tumour-invasion front (‘peritumoural’ budding, PTB) (Fig. 1b).1 As these entities can be distributed throughout the tumour mass, tumour budding is amenable to detection in surgical resections as well as by biopsy, which is potentially highly significant in the context of pre- and post-operative patient management for some tumour types. Tumour budding occurs in a large variety of cancers from different organs.2 The frequency of high-grade tumour budding is difficult to estimate, due to the use of various scoring systems, but it might be found in approximately 40% of colorectal cancers (CRC),3 oesophageal cancers and gastric cancers4,5,6,7,8 (Fig. 1c–e), as well as in more than 50% of pancreatic ductal adenocarcinomas (PDACs)9 (Fig. 1f) and cholangiocarcinomas.10 Tumour budding is also reported to occur in many other cancer types, such as head and neck cancers,11 lung adenocarcinomas and squamous cell carcinomas12,13 as well as breast14 and cervical15 cancers.
Intratumoural (ITB) (a) and peritumoral (PTB) (b) budding in colorectal cancer; tumour buds at the invasive front of oesophageal squamous cell cancer (c) and oesophageal adenocarcinoma (d), and tumour budding in gastric (e) and pancreatic ductal adenocarcinoma (f). Original images from cases at the Institute of Pathology, University of Bern, approved by the ethics committee of the canton of Bern (KEK Bern). Patients have signed written informed consent.
Evidence indicates that tumour buds might adopt the properties of cells undergoing epithelial–mesenchymal transition (EMT), suggesting that these cells have a more invasive and migratory potential.16 Double staining for the epithelial marker cytokeratin and the mesenchymal marker vimentin, highlights a small number of tumour buds that co-express both proteins, adding weight to the hypothesis that a ‘hybrid’ EMT phenotype exists in a subset of these cells.17 EMT has been linked to therapy resistance and cancer-cell stemness,18,19 so it follows that the detection of tumour budding in preoperative biopsy samples of patients with, for example, either rectal or oesophageal cancers, is associated with a poor response to neoadjuvant therapy and overall clinical outcome.20,21 Mounting data suggest that the presence of tumour budding is an unfavourable prognostic factor across all tumour types in which it is found and is tightly associated with lymph node metastasis, local recurrence and distant metastatic disease.
The reason for the occurrence of tumour budding is not known. DNA sequencing studies show no difference in the mutational profile of driver genes in tumour buds in comparison with the main tumour mass,22 although RNA sequencing studies clearly underline changes in mRNA and microRNAs involved in transforming growth factor-β (TGF-β) and WNT signalling pathways.3,23 Downstream of these pathways, repressors of the cell–cell adhesion molecule E-cadherin are overexpressed, as are markers of extracellular matrix degradation and migration. The presence of tumour buds in areas of desmoplastic stroma strongly suggests an interplay between tumour cells and cancer-associated fibroblasts, which presents an exciting area for future investigation.
Although first described in cancer of the lip,24 tumour budding has increased in popularity as an important prognostic factor in CRC. The first guideline for reporting tumour budding was published in 2017 for CRC following the 2016 International Tumour Budding Consensus Conference (ITBCC),1 and is now included in the College of American Pathologists (CAP) protocol. The ITBCC method was selected due to the large evidence base supporting its clinical utility, and is based on the Japanese Guidelines for Colorectal Cancer Reporting.25 The evidence-based guidelines describe clinical scenarios in which tumour budding should be assessed using a three-tier scoring system. In detail, the haematoxylin and eosin (H&E) slide with the greatest degree of budding at the invasion front (PTB) is selected, then ten individual fields at medium power (10× objective) are used to identify the ‘hotspot’ and tumour buds are counted using a ×20 objective. Normalisation of the count to an area of 0.785 mm2 (depending on the eyepiece field number) is performed, and the budding categories are defined: BD1 (1–4 buds), BD2 (5–9 buds, BD2) and BD3 (≥10 buds). Subsequent validation of these guidelines not only in CRC, but also in lung, gastric and PDAC, has underlined the usefulness of this standardised scoring approach.12,26,27 H&E is the standard stain, but pan-cytokeratin staining can also be used in conjunction with a number of approaches to assess tumour budding (Box 1).
In this review, we focus on presenting the latest data on budding in tumours of the gastrointestinal tract, giving an update on the clinical application of tumour budding in CRC and highlighting the latest data in the fields of oesophageal and gastric cancers, as well as PDAC and cholangiocarcinomas.
Clinical implications of tumour budding in CRC
In the era of personalised healthcare, the role of biomarkers is of immense importance. The ideal biomarker is prognostic and/or predictive, simple, reproducible and cost-effective and, therefore, only rarely are all these requirements fulfilled. Molecular biomarkers of the Ras signalling pathway, such as KRAS, HRAS, NRAS and BRAF, have been shown to play an important role in the pathogenesis of CRC;28 however, it should be kept in mind that the sum of all the molecular features probably leads to a particular morphological picture that has defined histopathological characteristics. One of those histological characteristics in CRC is tumour budding, a phenomenon that is indicative of tumour progression and adverse prognosis.29 There are now enough data in the literature, as well as the ITBCC guidelines, to justify implementing the assessment of tumour budding into routine clinical practice for CRC.30 In CRC, the presence of tumour budding along with other established biomarkers might support clinicians in four potential clinical scenarios (summarised in Table 1). First, PTB might indicate which patients benefit from oncological resection after the diagnosis of a primary tumour that has grown into the submucosa (pT1 CRC). Second, PTB in stage II CRC might indicate patients who should be considered for adjuvant therapy. Third, ITB can also be assessed in biopsy samples and therefore be included in the preoperative management, especially of rectal cancer patients who might undergo a neoadjuvant therapy. Fourth, in stage IV CRC patients, the presence of intrametastatic or perimetastatic tumour budding (IMB and PMB, respectively) in colorectal cancer liver metastases (CRLM) might be a supportive marker to stratify patients for different therapeutic options. The utility of tumour budding in patients with stage III cancers has not been evaluated in depth. However, as adjuvant therapy is the standard-of-care, the question arises as to whether tumour budding might be predictive of the response to chemotherapy in this subgroup of patients.
Clinical scenario 1: tumour budding in pT1 CRC
The clinical management of pT1 CRC includes the decision as to whether patients with early invasive cancer should undergo a wait-and-see approach or if they should be considered for oncological resection. There is therefore a major need for robust and reproducible biomarkers that correlate with the presence or absence of lymph node metastases. In 2004, Ueno et al. investigated a panel of clinicopathological parameters, including tumour location, tumour diameter, macroscopic tumour configuration (sessile vs. pedunculated), tumour grade, vascular invasion, tumour budding and width and depth of submucosal invasion.31 The study concluded that the absence of a number of features—including high tumour grade, vascular invasion, budding and extensive submucosal invasion—might potentially favour a watch-and-see policy.31 In 2013, Bosch et al. obtained similar results from a meta-analysis of 17 studies and 3782 patients with pT1 CRC.32 The results showed strong predictors for lymph node positivity to be submucosal invasion ≥1 mm (relative risk [RR] 5.2, 95% confidence interval [CI] 1.8–15.4), lymphatic invasion (RR 5.2, 95% CI 4.0–6.8), poor histological differentiation (RR 4.8, 95% CI 3.3–6.9) and tumour budding (RR 5.1, 95% CI 3.6–7.3). The conclusion was therefore quite similar to that proposed by Ueno et al.31—namely, that the absence of lymphatic invasion and budding, submucosal invasion ≤1 mm and poor histological differentiation was each associated with a low risk of lymph node metastases.32 In 2017, Cappellesso et al. focused specifically on the role of tumour budding in a meta-analysis of 41 studies and 10137 patients with pT1 CRC.33 They found tumour budding to be strongly associated with the risk of nodal metastases and, when comparing a positive tumour-budding status (684/2401, 28.5%) with a negative tumour-budding status (557/7736, 7.2%), the prevalence of lymph node positivity resulted in an odds ratio (OR) value of 6.44 (95% CI, 5.26–7.87, P = 0.0001; I2 = 30%, 41 studies).33 The ITBCC states that tumour budding is an independent predictor of lymph node metastases in pT1 CRC patients, and therefore strongly recommends that tumour budding is reported, along with other histopathological predictors of lymph node metastasis, such as poor differentiation, lymphovascular invasion and the depth/level of submucosal invasion,1 in patients with pT1 CRC.
Clinical scenario 2: tumour budding in stage II CRC
The updated European Society for Medical Oncology (ESMO) guidelines for the management and treatment recommends a follow-up for patients with low-risk stage II colon cancer, while adjuvant therapy with fluoropyrimidine should be considered for patients who have high-risk factors, such as T4 (the tumour has grown through all layers of the colon and attached to or invaded other structures and organs), number of examined lymph nodes <12, primary tumour perforation or occlusion, tumour grade 3 or absence of microsatellite instability (MSI).34,35 In the past 10 years, numerous studies and meta-analyses have reported tumour budding to be an independent factor of poor survival and recurrence in patients with stage II CRC, with outcomes similar to those of patients with stage III CRC.36,37,38,39,40,41,42,43,44 The ITBCC therefore recommended in 2016 that tumour budding be included among the high-risk factors in stage II CRC.1 This recommendation was supported by the 2019 World Health Organisation (WHO) classification of tumours of the digestive system, which reports tumour budding—along with perineural invasion, intramural and extramural vascular invasion, lymphatic invasion and tumour deposits—as a high-risk factor with an OR of 4.51 (95% CI, 2.55–7.99).45 In 2019, Ueno et al. validated the ITBCC scoring system in a multicentre stage II colon cancer cohort from 123 institutions (n = 991).46 The 5-year relapse-free survival (RFS) rate was 90.9% in patients with tumours classified as BD1, 85.1% in those with BD2 and 74.4% in those with BD3 (P < 0.001). There also was a significant correlation between the budding grade and recurrence in the liver, lungs, lymph nodes and peritoneum (P < 0.001–0.01). Multivariable analysis revealed that budding had an independent impact on RFS. The study concluded that tumour budding should be routinely reported in stage II colon cancer.46
Clinical scenario 3: tumour budding in CRC preoperative biopsy samples
In 1989, Morodomi et al. described the presence of tumour buds in biopsy samples from patients with rectal cancer and the association of this phenomenon with lymph node metastases.47 This observation led to a systematic assessment of PTB and ITB,48 and to the finding of a potential prognostic and predictive role for ITB. ITB is highly associated with PTB, and therefore a surrogate for the tumour-budding status of the whole tumour, as well as being associated with lymph node and distant metastases, local recurrence, poor survival and tumour regression grade.49,50,51,52,53 Therefore, the assessment of ITB in biopsy samples might have important clinical implications, especially in the preoperative management of rectal cancer patients. Patients who present with high-grade ITB along with the already-implemented clinical factors in preoperative biopsy samples might be considered for neoadjuvant therapy. Although the ITBCC recognises the ITB approach, more data are definitely necessary prior to its implementation in daily practice.1
Clinical scenario 4: tumour budding in CRLM
The ESMO consensus guidelines for the management of patients with metastatic CRC highlight the importance of a multidisciplinary management, including oncology, surgery, radiology and pathology.54 The most frequently used biomarkers in clinical practice are molecular, and report on the RAS, BRAF and MSI status,54 whereas current histopathological features reported for clinical management are metastatic size, percentage of fibrosis and necrosis, resection status and tumour regression grade.55 Several studies have shown the prognostic potential of the histological growth pattern—desmoplastic, pushing and replacement—of the tumour–liver interface of CRLM.56,57,58,59,60,61,62 Tumour budding, a morphological feature of the tumour microenvironment at the invasive front, might therefore also be an important factor in disease progression in stage IV CRC patients. Similar to the primary tumour, tumour buds can be detected at the invasive front (PMB) or within the main metastasis body (IMB)63 but, in contrast to the primary tumour, there is still a major challenge for scoring tumour budding in CRLM. Indeed, the detection of tumour buds can be difficult in cases without desmoplastic stromal reaction or a strong reactive perimetastatic ductular proliferation.63 In addition, pan-cytokeratin immunohistochemical staining can sometimes obscure important morphological features, and therefore a budding score based on H&E staining is recommended.63 In a 2018 study, tumour budding assessed in CRLM from 229 patients with stage IV CRC was a prognostic factor, but not an independent predictor of survival.64
In summary, there are currently not enough data to make any firm conclusions on the prognostic or predictive role of tumour budding in CRLM, and further retrospective and prospective studies on large multicentric cohorts are needed.
Clinical implications of tumour budding in oesophageal and gastric cancer
The first study on tumour budding in oesophageal squamous cell carcinoma (SCC) dates from the early 2000s. Investigating tumour budding by H&E in a small cohort of 56 patients, which included surgically treated individuals with stage I–III oesophageal SCC, Roh et al. found a marked reduction in the 3-year survival rate in patients with high-grade versus low-grade budding (30.7% vs. 72.3%, respectively).65 Similar results have been observed in numerous studies (Koike et al.,66 Miyata et al.21 and Teramoto et al.,67 Jesinghaus et al.,68,69 Niwa et al.70 and Ito et al.71) underlining significantly poorer 3-year or 5-year survival rates after oesophagectomy in SCC patients with high-grade budding. Nakanishi and colleagues published comparable results, with 5-year survival rates of 49% versus 15% in 74 patients with low-grade versus high-grade budding, respectively, receiving preoperative chemotherapy.72 The results of these studies have been reviewed by Koelzer et al.73 High-grade tumour budding has also been found to be associated with lymph node metastasis in oesophageal lesions involving the muscularis mucosae (T1a-MM) to those of the upper third of the submucosa (T1b-SM1) using both H&E and cytokeratin staining.74 These results suggest that budding could be a useful histomorphological feature in patients with primary resected oesophageal SCC in the neoadjuvant setting and in early-stage cancers.
Although only a handful of studies have evaluated tumour budding in oesophageal adenocarcinoma, similar results have been reported.73 High-grade tumour budding is described in 28–51.7% of cases, albeit using different scoring systems and based on either H&E or pan-cytokeratin staining.75 Its presence is associated with higher TNM stage, lymph node metastases, poor disease-free survival and poor overall survival. These results again highlight the correlation between tumour budding and disease course, and might be useful to guide follow-up.
Guo and colleagues published a review summarising the evidence of tumour budding in gastric cancer in 2019.7 Seven cohorts encompassing data from 2178 patients were analysed; the method used to analyse budding was based on H&E staining, and the cut-off values for ‘high-grade’ budding varied across studies, from five or ten buds to the median number of buds in the particular cohort. As a first step, the presence of high-grade tumour budding showed a positive correlation with tumour stage (OR 6.63, 95% CI 4.01–10.98, P < 0.0001) as well as with undifferentiated tumour status (OR 3.74, 95% CI 2.68–5.22, P < 0.01). High-grade tumour budding was significantly associated with lymphatic vessel invasion and lymph node metastasis (OR 7.85, 95% CI 5.04–12.21, P < 0.01, and OR 5.75, 95% CI 3.20–10.32, P < 0.01, respectively), as well as with poor 5-year overall survival in a pooled analysis of 1833 patients (HR 1.79, 95% CI 1.53–2.05, P < 0.01). These results were confirmed in a subgroup analysis of intestinal-type cancers but not in diffuse-type adenocarcinoma.76 In their evaluation of tumour budding in 621 radical gastrectomies for submucosal early gastric carcinoma,77 Du et al. identified high-grade tumour budding as a predictor of lymph node metastases (OR 3.3, 95% CI 1.9–5.9). Moreover, when Ulase et al. applied the ITBCC tumour-budding score to 456 surgically resected gastric cancers, they found that the BD score was significantly associated with sex, Laurén phenotype, pT-, pN- and pM classification, as well as perineural invasion and survival times.26
In summary, tumour budding shows prognostic potential in oesophageal adenocarcinomas, SCC and gastric cancers, and might be predictive in the neoadjuvant setting. Although a standardised scoring system is still missing, the ITBCC approach for CRC might also be applicable in the context of upper gastrointestinal cancers.
Clinical implications of tumour budding in cholangiocarcinoma and pancreatic ductal adenocarcinoma
Cholangiocarcinoma is a rare type of tumour, comprising <1% of all cancers;78 it is subdivided into intrahepatic and extrahepatic cholangiocarcinoma, and the latter can be further subclassified as perihilar or distal cholangiocarcinoma.79 The vast majority of patients with cholangiocarcinoma present with unresectable disease at the time of diagnosis. Consequently, the prognosis for cholangiocarcinoma is poor, with a 5-year OS of 30–40% for localised tumours and a median OS of nearly 12 months for unresectable or metastatic disease.80,81
Although tumour budding is a well-established prognostic factor in CRC,82 its significance in cholangiocarcinoma is far less clear. However, Ogino et al. demonstrated in 2019 that peritumoral budding in both perihilar and extrahepatic cholangiocarcinoma is associated with adverse clinicopathological features, such as higher T stage, lymphovascular and perineural invasion, lymph node metastases and higher histological grade, which translate into a worse clinical outcome.83 Cholangiocarcinoma patients with high-grade tumour budding had a significantly shorter OS compared with those with low-grade tumour budding. In another cholangiocarcinoma cohort comprising 299 Asian patients, the presence of peritumoral budding was associated with worse OS.84 According to the results of these two retrospective studies, tumour budding might be a potential prognostic factor.
Based on the results of the adjusted intention-to-treat and per-protocol analysis of the previously published BILCAP trial, a randomised clinical trial comparing adjuvant chemotherapy with capecitabine with expectant treatment following resection, the American Society of Clinical Oncology (ASCO) guidelines recommended adjuvant treatment with capecitabine in resected cholangiocarcinoma patients.85,86 However, the study population was heterogeneous, comprising all T and N stages with or without R0 resection. A preplanned subanalysis indicated that male patients and those with poorly differentiated tumours derived the most benefit from adjuvant capecitabine treatment,83,85 highlighting the importance of identifying predictive biomarkers to predict which subset of patients with resected cholangiocarcinoma should respond to chemotherapy. Further validation in independent datasets—preferably from Phase 3 studies—is needed to finally confirm both the prognostic and predictive values of tumour budding in cholangiocarcinoma. In addition, no data demonstrating whether patients with high-grade, tumour-budding cholangiocarcinoma might benefit from adjuvant chemotherapy exist yet. Therefore, the predictive impact of tumour budding in cholangiocarcinonoma is still unclear.
PDAC also has a poor prognosis, with a 5-year OS of ~5%.87 In 2019, a meta-analysis demonstrated that patients with PDAC exhibiting high-grade tumour budding had a higher all-cause mortality rate compared with those who showed low-grade tumour budding (HR 2.65, 95% CI 1.79–3.91, P < 0.0001).88 Due to the aggressive tumour biology and the inherent poor prognosis of PDAC, adjuvant chemotherapy with either FOLFIRINOX (folinic acid, fluorouracil, irinotecan and oxaliplatin) or gemcitabine combined with capecitabine is recommended in all patients after curative resected PDAC, regardless of the pathological stage.89,90 However, patients who may not qualify for a doublet or triplet therapy, treatment with gemcitabine alone is a reasonable option. In a retrospective analysis of the CONKO-001 trial, designed to compare adjuvant gemcitabine with observation in patients with PDAC undergoing complete, curative-intent tumour resection, the presence of tumour budding was associated with decreased OS, irrespective of whether or not the patients were treated with adjuvant gemcitabine.91 In this study, no further subclassification into PTB or ITB has been carried out. In contrast to the situation for stage II colon cancer, in which, although still unproven, the presence of high-grade tumour budding might contribute to the treatment strategy, tumour budding in PDAC has not yet had an impact on adjuvant treatment decisions. However, just as new treatment strategies targeting PDAC might continue to evolve in the near future, the role of tumour budding on clinical decision-making might be redefined.
Perspectives for tumour budding in gastrointestinal cancers
Tumour budding is emerging as a promising morphological biomarker not only in CRC, but also in other gastrointestinal cancers, such as oesophageal, gastric, PDAC and cholangiocarcinomas2,73,83 (the prognostic and/or predictive value of tumour budding in non-CRC gastrointestinal cancers is summarised in Table 2). An interesting observation is the fact that tumour budding can be detected in adenocarcinomas and in SCCs, as these morphological tumour subtypes have different criteria for dedifferentiation (solid areas vs. keratinisation), which is highlighted by many studies investigating the clinical implication of tumour budding in oral cavity cancers.92 Nevertheless, one should keep in mind that, depending on the tumour type, the definition and scoring system for tumour budding might differ, similar to the tumour gradings published by the Union for International Cancer Control (UICC), American Joint Committee on Cancer (AJCC) and the WHO. Grading in SCC of the oesophagus is based on the degree of cytological atypia, mitotic activity and the presence of keratinisation, whereas in colorectal adenocarcinoma, grading is determined according to solid areas.
For CRC, four aspects of tumour budding need to be addressed in order to optimise its clinical application. Interobserver variability in the assessment of budding is still suboptimal, especially among non-gastrointestinal pathologists, leading potentially to the up- or downgrading of budding. Accordingly, tumour-budding courses are needed to improve the reproducibility of tumour budding in CRC, similar to the assessment by immunohistochemistry of programmed death ligand 1 (PD-L1) in lung cancer.30,93,94 Alternatively, the development of a digitally supported scoring system for tumour budding could be time-saving as well as beneficial for increasing reproducibility.95,96 In addition, although enough data exist for tumour budding in pT1 CRC and stage II CRC patients for implementation into clinical practice, more studies are needed for the clinical scenarios that involve preoperative biopsies and the assessment of tumour budding in CRLM. Finally, although the predictive value of tumour budding is still not clear, the investigation of potential target molecules expressed by tumour buds might offer a promising approach for an anti-budding therapy to specifically target the tumour cells that seem to be responsible for local and distant metastases and consequently for tumour progression and decreased survival.
References
Lugli, A., Kirsch, R., Ajioka, Y., Bosman, F., Cathomas, G., Dawson, H. et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 30, 1299–1311 (2017).
Berg, K. B. & Schaeffer, D. F. Tumor budding as a standardized parameter in gastrointestinal carcinomas: more than just the colon. Mod. Pathol. 31, 862–872 (2018).
De Smedt, L., Palmans, S., Andel, D., Govaere, O., Boeckx, B., Smeets, D. et al. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br. J. Cancer 116, 58–65 (2017).
Davison, J. M., Landau, M. S., Luketich, J. D., McGrath, K. M., Foxwell, T. J., Landsittel, D. P. et al. A model based on pathologic features of superficial esophageal adenocarcinoma complements clinical node staging in determining risk of metastasis to lymph nodes. Clin. Gastroenterol. Hepatol. 14, 369–77 e3 (2016).
Almangush, A., Karhunen, M., Hautaniemi, S., Salo, T. & Leivo, I. Prognostic value of tumour budding in oesophageal cancer: a meta-analysis. Histopathology 68, 173–182 (2016).
Thies, S., Guldener, L., Slotta-Huspenina, J., Zlobec, I., Koelzer, V. H., Lugli, A. et al. Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas. Hum. Pathol. 52, 1–8 (2016).
Guo, Y. X., Zhang, Z. Z., Zhao, G. & Zhao, E. H. Prognostic and pathological impact of tumor budding in gastric cancer: a systematic review and meta-analysis. World J. Gastrointest. Oncol. 11, 898–908 (2019).
Gulluoglu, M., Yegen, G., Ozluk, Y., Keskin, M., Dogan, S., Gundogdu, G. et al. Tumor budding is independently predictive for lymph node involvement in early gastric cancer. Int J. Surg. Pathol. 23, 349–358 (2015).
Karamitopoulou, E., Wartenberg, M., Zlobec, I., Cibin, S., Worni, M., Gloor, B. et al. Tumour budding in pancreatic cancer revisited: validation of the ITBCC scoring system. Histopathology 73, 137–146 (2018).
Tanaka, M., Yamauchi, N., Ushiku, T., Shibahara, J., Hayashi, A., Misumi, K. et al. Tumor budding in intrahepatic cholangiocarcinoma: a predictor of postsurgery outcomes. Am. J. Surg. Pathol. 43, 1180–1190 (2019).
Ho, Y. Y., Wu, T. Y., Cheng, H. C., Yang, C. C. & Wu, C. H. The significance of tumor budding in oral cancer survival and its relevance to theeighth edition of the American Joint Committee on Cancer staging system. Head Neck 41, 2991–3001 (2019).
Neppl, C., Zlobec, I., Schmid, R. A. & Berezowska, S. Validation of the International Tumor Budding Consensus Conference (ITBCC) 2016 recommendation in squamous cell carcinoma of the lung-a single-center analysis of 354 cases. Mod. Pathol. 33, 802–811 (2020).
Kadota, K., Yeh, Y. C., Villena-Vargas, J., Cherkassky, L., Drill, E. N., Sima, C. S. et al. Tumor budding correlates with the protumor immune microenvironment and is an independent prognostic factor for recurrence of stage I lung adenocarcinoma. Chest 148, 711–721 (2015).
Li, X., Wei, B., Sonmez, C., Li, Z. & Peng, L. High tumor budding count is associated with adverse clinicopathologic features and poor prognosis in breast carcinoma. Hum. Pathol. 66, 222–229 (2017).
Jesinghaus, M., Strehl, J., Boxberg, M., Bruhl, F., Wenzel, A., Konukiewitz, B. et al. Introducing a novel highly prognostic grading scheme based on tumour budding and cell nest size for squamous cell carcinoma of the uterine cervix. J. Pathol. Clin. Res. 4, 93–102 (2018).
Meyer, S. N., Galvan, J. A., Zahnd, S., Sokol, L., Dawson, H., Lugli, A. et al. Co-expression of cytokeratin and vimentin in colorectal cancer highlights a subset of tumor buds and an atypical cancer-associated stroma. Hum. Pathol. 87, 18–27 (2019).
Grigore, A. D., Jolly, M. K., Jia, D., Farach-Carson, M. C. & Levine, H. Tumor budding: the name is EMT. Partial EMT. J. Clin. Med. 5, 51 (2016).
Maffeis, V., Nicole, L. & Cappellesso, R. RAS, cellular plasticity, and tumor budding in colorectal cancer. Front Oncol. 9, 1255 (2019).
Steinbichler, T. B., Savic, D., Dudas, J., Kvitsaridze, I., Skvortsov, S., Riechelmann, H. et al. Cancer stem cells and their unique role in metastatic spread. Semin Cancer Biol. 60, 148–156 (2020).
Jager, T., Neureiter, D., Fallaha, M., Schredl, P., Kiesslich, T., Urbas, R. et al. The potential predictive value of tumor budding for neoadjuvant chemoradiotherapy response in locally advanced rectal cancer. Strahlenther. Onkol. 194, 991–1006 (2018).
Miyata, H., Yoshioka, A., Yamasaki, M., Nushijima, Y., Takiguchi, S., Fujiwara, Y. et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer 115, 3324–3334 (2009).
Centeno, I., Paasinen Sohns, A., Flury, M., Galvan, J. A., Zahnd, S., Koelzer, V. H. et al. DNA profiling of tumor buds in colorectal cancer indicates that they have the same mutation profile as the tumor from which they derive. Virchows Arch. 470, 341–346 (2017).
Jensen, D. H., Dabelsteen, E., Specht, L., Fiehn, A. M., Therkildsen, M. H., Jonson, L. et al. Molecular profiling of tumour budding implicates TGFbeta-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma. J. Pathol. 236, 505–516 (2015).
Broders, A. C. Squamous-cell epithelioma of the lip. A study of five hundred and thirty-seven cases. J. Am. Med. Assoc. 74, 656–664 (1920).
Watanabe, T., Muro, K., Ajioka, Y., Hashiguchi, Y., Ito, Y., Saito, Y. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J. Clin. Oncol. 23, 1–34 (2018).
Ulase, D., Heckl, S., Behrens, H. M., Kruger, S. & Rocken, C. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology 76, 433–446 (2020).
Petrova, E., Zielinski, V., Bolm, L., Schreiber, C., Knief, J., Thorns, C. et al. Tumor budding as a prognostic factor in pancreatic ductal adenocarcinoma. Virchows Arch. 476, 561–568 (2020).
Pritchard, C. C. & Grady, W. M. Colorectal cancer molecular biology moves into clinical practice. Gut 60, 116–129 (2011).
Rogers, A. C., Winter, D. C., Heeney, A., Gibbons, D., Lugli, A., Puppa, G. et al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 115, 831–840 (2016).
Cho, S. J. & Kakar, S. Tumor budding in colorectal carcinoma: translating a morphologic score into clinically meaningful results. Arch. Pathol. Lab Med. 142, 952–957 (2018).
Ueno, H., Mochizuki, H., Hashiguchi, Y., Shimazaki, H., Aida, S., Hase, K. et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 127, 385–394 (2004).
Bosch, S. L., Teerenstra, S., de Wilt, J. H., Cunningham, C. & Nagtegaal, I. D. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 45, 827–834 (2013).
Cappellesso, R., Luchini, C., Veronese, N., Lo Mele, M., Rosa-Rizzotto, E., Guido, E. et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum. Pathol. 65, 62–70 (2017).
van de Velde, C. J., Boelens, P. G., Borras, J. M., Coebergh, J. W., Cervantes, A., Blomqvist, L. et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur. J. Cancer 50, 1 e–e34 (2014).
Schmoll, H. J., Van Cutsem, E., Stein, A., Valentini, V., Glimelius, B., Haustermans, K. et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 23, 2479–2516 (2012).
Betge, J., Kornprat, P., Pollheimer, M. J., Lindtner, R. A., Schlemmer, A., Rehak, P. et al. Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann. Surg. Oncol. 19, 3706–3712 (2012).
Canney, A. L., Kevans, D., Wang, L. M., Hyland, J. M., Mulcahy, H. E., O'Donoghue, D. P. et al. Stage II colonic adenocarcinoma: a detailed study of pT4N0 with emphasis on peritoneal involvement and the role of tumour budding. Histopathology 61, 488–496 (2012).
Petrelli, F., Pezzica, E., Cabiddu, M., Coinu, A., Borgonovo, K., Ghilardi, M. et al. Tumour budding and survival in stage II colorectal cancer: a systematic review and pooled analysis. J. Gastrointest. Cancer 46, 212–218 (2015).
Lai, Y. H., Wu, L. C., Li, P. S., Wu, W. H., Yang, S. B., Xia, P. et al. Tumour budding is a reproducible index for risk stratification of patients with stage II colon cancer. Colorectal Dis. 16, 259–264 (2014).
Nakamura, T., Mitomi, H., Kanazawa, H., Ohkura, Y. & Watanabe, M. Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis. Colon Rectum 51, 568–572 (2008).
Hayes, B. D., Maguire, A., Conlon, N., Gibbons, D., Wang, L. M. & Sheahan, K. Reproducibility of the rapid bud count method for assessment of tumor budding in stage II colorectal cancer. Am. J. Surg. Pathol. 34, 746–748 (2010).
Okuyama, T., Nakamura, T. & Yamaguchi, M. Budding is useful to select high-risk patients in stage II well-differentiated or moderately differentiated colon adenocarcinoma. Dis. Colon Rectum 46, 1400–1406 (2003).
Okuyama, T., Oya, M. & Ishikawa, H. Budding as a risk factor for lymph node metastasis in pT1 or pT2 well-differentiated colorectal adenocarcinoma. Dis. Colon Rectum 45, 628–634 (2002).
Okuyama, T., Oya, M. & Ishikawa, H. Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J. Surg. Oncol. 83, 42–47 (2003).
WHO Classification of Tumours Editorial Board. Digestive System Tumours, Fifth edn. (IARC, Lyon, France, 2019).
Ueno, H., Ishiguro, M., Nakatani, E., Ishikawa, T., Uetake, H., Matsuda, C. et al. Prospective multicenter study on the prognostic and predictive impact of tumor budding in stage II colon cancer: results from the SACURA trial. J. Clin. Oncol. 37, 1886–1894 (2019).
Morodomi, T., Isomoto, H., Shirouzu, K., Kakegawa, K., Irie, K. & Morimatsu, M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 63, 539–543 (1989).
Lugli, A., Vlajnic, T., Giger, O., Karamitopoulou, E., Patsouris, E. S., Peros, G. et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum. Pathol. 42, 1833–1840 (2011).
Zlobec, I., Hadrich, M., Dawson, H., Koelzer, V. H., Borner, M., Mallaev, M. et al. Intratumoural budding (ITB) in preoperative biopsies predicts the presence of lymph node and distant metastases in colon and rectal cancer patients. Br. J. Cancer 110, 1008–1013 (2014).
Rogers, A. C., Gibbons, D., Hanly, A. M., Hyland, J. M., O'Connell, P. R., Winter, D. C. et al. Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod. Pathol. 27, 156–162 (2014).
Giger, O. T., Comtesse, S. C., Lugli, A., Zlobec, I. & Kurrer, M. O. Intra-tumoral budding in preoperative biopsy specimens predicts lymph node and distant metastasis in patients with colorectal cancer. Mod. Pathol. 25, 1048–1053 (2012).
Lino-Silva, L. S., Gamboa-Dominguez, A., Zuniga-Tamayo, D., Salcedo-Hernandez, R. A., Cetina, L. & Cantu-de-Leon, D. Mismatch repair protein expression and intratumoral budding in rectal cancer are associated with an increased pathological complete response to preoperative chemoradiotherapy: a case-control study. World J. Clin. Oncol. 9, 133–139 (2018).
Marx, A. H., Mickler, C., Sauter, G., Simon, R., Terracciano, L. M., Izbicki, J. R. et al. High-grade intratumoral tumor budding is a predictor for lymphovascular invasion and adverse outcome in stage II colorectal cancer. Int. J. Colorectal Dis. 35, 259–268 (2020).
Van Cutsem, E., Cervantes, A., Adam, R., Sobrero, A., Van Krieken, J. H., Aderka, D. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 27, 1386–1422 (2016).
Rubbia-Brandt, L., Giostra, E., Brezault, C., Roth, A. D., Andres, A., Audard, V. et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 18, 299–304 (2007).
Van den Eynden, G. G., van Dam, P. J., Stroobants, S., Dirix, L., Vermeulen, P. & Liver Metastasis Research, N. Histopathological evaluation of resected colorectal cancer liver metastases: what should be done? Histopathology 64, 315–316 (2014).
Van den Eynden, G. G., Majeed, A. W., Illemann, M., Vermeulen, P. B., Bird, N. C., Hoyer-Hansen, G. et al. The multifaceted role of the microenvironment in liver metastasis: biology and clinical implications. Cancer Res. 73, 2031–2043 (2013).
Van den Eynden, G. G., Bird, N. C., Majeed, A. W., Van Laere, S., Dirix, L. Y. & Vermeulen, P. B. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis. 29, 541–549 (2012).
Lazaris, A., Amri, A., Petrillo, S. K., Zoroquiain, P., Ibrahim, N., Salman, A. et al. Vascularization of colorectal carcinoma liver metastasis: insight into stratification of patients for anti-angiogenic therapies. J. Pathol. Clin. Res. 4, 184–192 (2018).
Frentzas, S., Simoneau, E., Bridgeman, V. L., Vermeulen, P. B., Foo, S., Kostaras, E. et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 22, 1294–1302 (2016).
Eefsen, R. L., Vermeulen, P. B., Christensen, I. J., Laerum, O. D., Mogensen, M. B., Rolff, H. C. et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin. Exp. Metastasis. 32, 369–381 (2015).
van Dam, P. J., van der Stok, E. P., Teuwen, L. A., Van den Eynden, G. G., Illemann, M., Frentzas, S. et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br. J. Cancer 117, 1427–1441 (2017).
Blank, A., Schenker, C., Dawson, H., Beldi, G., Zlobec, I. & Lugli, A. Evaluation of tumor budding in primary colorectal cancer and corresponding liver metastases based on H&E and pancytokeratin staining. Front Med. 6, 247 (2019).
Fonseca, G. M., de Mello, E. S., Faraj, S. F., Kruger, J. A. P., Coelho, F. F., Jeismann, V. B. et al. Prognostic significance of poorly differentiated clusters and tumor budding in colorectal liver metastases. J. Surg. Oncol. 117, 1364–1375 (2018).
Roh, M. S., Lee, J. I. & Choi, P. J. Tumor budding as a useful prognostic marker in esophageal squamous cell carcinoma. Dis. Esophagus 17, 333–337 (2004).
Koike, M., Kodera, Y., Itoh, Y., Nakayama, G., Fujiwara, M., Hamajima, N. et al. Multivariate analysis of the pathologic features of esophageal squamous cell cancer: tumor budding is a significant independent prognostic factor. Ann. Surg. Oncol. 15, 1977–1982 (2008).
Teramoto, H., Koike, M., Tanaka, C., Yamada, S., Nakayama, G., Fujii, T. et al. Tumor budding as a useful prognostic marker in T1-stage squamous cell carcinoma of the esophagus. J. Surg. Oncol. 108, 42–46 (2013).
Jesinghaus, M., Boxberg, M., Konukiewitz, B., Slotta-Huspenina, J., Schlitter, A. M., Steiger, K. et al. A novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am. J. Surg. Pathol. 41, 1112–1120 (2017).
Jesinghaus, M., Bruhl, F., Steiger, K., Klare, P., Reiser, M., Scheiter, A. et al. Cellular dissociation grading based on the parameters tumor budding and cell nest size in pretherapeutic biopsy specimens allows for prognostic patient stratification in esophageal squamous cell carcinoma independent from clinical staging. Am. J. Surg. Pathol. 43, 618–627 (2019).
Niwa, Y., Yamada, S., Koike, M., Kanda, M., Fujii, T., Nakayama, G. et al. Epithelial to mesenchymal transition correlates with tumor budding and predicts prognosis in esophageal squamous cell carcinoma. J. Surg. Oncol. 110, 764–769 (2014).
Ito, E., Ozawa, S., Kijima, H., Kazuno, A., Nishi, T., Chino, O. et al. New invasive patterns as a prognostic factor for superficial esophageal cancer. J. Gastroenterol. 47, 1279–1289 (2012).
Nakanishi, Y., Ohara, M., Doumen, H., Kimura, N., Ishidate, T. & Kondo, S. Correlation between tumor budding and post-resection prognosis in patients with invasive squamous cell carcinoma of the thoracic esophagus. World J. Surg. 35, 349–356 (2011).
Koelzer, V. H., Langer, R., Zlobec, I. & Lugli, A. Tumor budding in upper gastrointestinal carcinomas. Front. Oncol. 4, 216 (2014).
Fuchinoue, K., Nemoto, T., Shimada, H., Tochigi, N., Igarashi, Y., Yajima, S. et al. Immunohistochemical analysis of tumor budding as predictor of lymph node metastasis from superficial esophageal squamous cell carcinoma. Esophagus 17, 168–174 (2020).
Landau, M. S., Hastings, S. M., Foxwell, T. J., Luketich, J. D., Nason, K. S. & Davison, J. M. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophageal adenocarcinoma. Mod. Pathol. 27, 1578–1589 (2014).
Kemi, N., Eskuri, M., Ikalainen, J., Karttunen, T. J. & Kauppila, J. H. Tumor budding and prognosis in gastric adenocarcinoma. Am. J. Surg. Pathol. 43, 229–234 (2019).
Du, M., Chen, L., Cheng, Y., Wang, Y., Fan, X., Zhang, Y. et al. Tumor budding and other risk factors of lymph node metastasis in submucosal early gastric carcinoma: a multicenter clinicopathologic study in 621 radical gastrectomies of Chinese patients. Am. J. Surg. Pathol. 43, 1074–1082 (2019).
Shaib, Y. & El-Serag, H. B. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 24, 115–125 (2004).
Khan, S. A., Toledano, M. B. & Taylor-Robinson, S. D. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB 10, 77–82 (2008).
Rea, D. J., Munoz-Juarez, M., Farnell, M. B., Donohue, J. H., Que, F. G., Crownhart, B. et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch. Surg. 139, 514–523 (2004).
Valle, J., Wasan, H., Palmer, D. H., Cunningham, D., Anthoney, A., Maraveyas, A. et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 362, 1273–1281 (2010).
Dawson, H., Galuppini, F., Trager, P., Berger, M. D., Studer, P., Brugger, L. et al. Validation of the International Tumor Budding Consensus Conference 2016 recommendations on tumor budding in stage I-IV colorectal cancer. Hum. Pathol. 85, 145–151 (2019).
Ogino, M., Nakanishi, Y., Mitsuhashi, T., Hatanaka, Y., Amano, T., Marukawa, K. et al. Impact of tumour budding grade in 310 patients who underwent surgical resection for extrahepatic cholangiocarcinoma. Histopathology 74, 861–872 (2019).
Okubo, S., Mitsunaga, S., Kato, Y., Kojima, M., Sugimoto, M., Gotohda, N. et al. The prognostic impact of differentiation at the invasive front of biliary tract cancer. J. Surg. Oncol. 117, 1278–1287 (2018).
Primrose, J. N., Fox, R. P., Palmer, D. H., Malik, H. Z., Prasad, R., Mirza, D. et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 20, 663–673 (2019).
Shroff, R. T., Kennedy, E. B., Bachini, M., Bekaii-Saab, T., Crane, C., Edeline, J. et al. Adjuvant therapy for resected biliary tract cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 37, 1015–1027 (2019).
Ducreux, M., Cuhna, A. S., Caramella, C., Hollebecque, A., Burtin, P., Goere, D. et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 26(Suppl 5), v56–v68 (2015).
Lawlor, R. T., Veronese, N., Nottegar, A., Malleo, G., Smith, L., Demurtas, J. et al. Prognostic role of high-grade tumor budding in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis with a focus on epithelial to mesenchymal transition. Cancers (Basel). 11, 113 (2019).
Neoptolemos, J. P., Palmer, D. H., Ghaneh, P., Psarelli, E. E., Valle, J. W., Halloran, C. M. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Conroy, T., Hammel, P., Hebbar, M., Ben Abdelghani, M., Wei, A. C., Raoul, J. L. et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 379, 2395–2406 (2018).
Lohneis, P., Sinn, M., Klein, F., Bischoff, S., Striefler, J. K., Wislocka, L. et al. Tumour buds determine prognosis in resected pancreatic ductal adenocarcinoma. Br. J. Cancer 118, 1485–1491 (2018).
Almangush, A., Pirinen, M., Heikkinen, I., Makitie, A. A., Salo, T. & Leivo, I. Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br. J. Cancer 118, 577–586 (2018).
Kai, K., Aishima, S., Aoki, S., Takase, Y., Uchihashi, K., Masuda, M. et al. Cytokeratin immunohistochemistry improves interobserver variability between unskilled pathologists in the evaluation of tumor budding in T1 colorectal cancer. Pathol. Int. 66, 75–82 (2016).
Martin, B., Schafer, E., Jakubowicz, E., Mayr, P., Ihringer, R., Anthuber, M. et al. Interobserver variability in the H&E-based assessment of tumor budding in pT3/4 colon cancer: does it affect the prognostic relevance? Virchows Arch. 473, 189–197 (2018).
Bokhorst, J. M., Blank, A., Lugli, A., Zlobec, I., Dawson, H., Vieth, M. et al. Assessment of individual tumor buds using keratin immunohistochemistry: moderate interobserver agreement suggests a role for machine learning. Mod. Pathol. 33, 825–833 (2020).
Fauzi, M. F. A., Chen, W., Knight, D., Hampel, H., Frankel, W. L. & Gurcan, M. N. Tumor budding detection system in whole slide pathology images. J. Med Syst. 44, 38 (2019).
Lugli, A., Karamitopoulou, E. & Zlobec, I. Tumour budding: a promising parameter in colorectal cancer. Br. J. Cancer 106, 1713–1717 (2012).
Koelzer, V. H., Zlobec, I. & Lugli, A. Tumor budding in colorectal cancer–ready for diagnostic practice? Hum. Pathol. 47, 4–19 (2016).
Che, K., Zhao, Y., Qu, X., Pang, Z., Ni, Y., Zhang, T. et al. Prognostic significance of tumor budding and single cell invasion in gastric adenocarcinoma. Onco Targets Ther. 10, 1039–1047 (2017).
Tanaka, K., Shimura, T., Kitajima, T., Kondo, S., Ide, S., Okugawa, Y. et al. Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br. J. Cancer 110, 2923–2934 (2014).
Gabbert, H. E., Meier, S., Gerharz, C. D. & Hommel, G. Tumor-cell dissociation at the invasion front: a new prognostic parameter in gastric cancer patients. Int. J. Cancer 50, 202–207 (1992).
Kohler, I., Bronsert, P., Timme, S., Werner, M., Brabletz, T., Hopt, U. T. et al. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 30(Suppl 1), 78–84 (2015).
O'Connor, K., Li-Chang, H. H., Kalloger, S. E., Peixoto, R. D., Webber, D. L., Owen, D. A. et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 39, 472–478 (2015).
Karamitopoulou, E., Zlobec, I., Born, D., Kondi-Pafiti, A., Lykoudis, P., Mellou, A. et al. Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur. J. Cancer 49, 1032–1039 (2013).
Author information
Authors and Affiliations
Contributions
All authors participated in the concept, drafting, editing and revision of this paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Images in Fig. 1 are from cases at the Institute of Pathology, University of Bern, approved by the ethics committee of the canton of Bern (KEK Bern). Patients have signed written informed consent.
Consent to publish
Not applicable.
Data availability
All data are included in this published article.
Competing interests
The authors declare no competing interests.
Funding information
Not applicable.
Additional information
Note This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution 4.0 International (CC BY 4.0).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zlobec, I., Berger, M.D. & Lugli, A. Tumour budding and its clinical implications in gastrointestinal cancers. Br J Cancer 123, 700–708 (2020). https://doi.org/10.1038/s41416-020-0954-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-020-0954-z
This article is cited by
-
P4HA2 involved in SLUG-associated EMT predicts poor prognosis of patients with KRAS-positive colorectal cancer
Medical Molecular Morphology (2024)
-
Role of Tumour Budding in Breast Cancer and Its Correlation with Other Prognostic Factors: A Cross Sectional Study in Rural Central India
Indian Journal of Gynecologic Oncology (2024)
-
The radiomorphological appearance of the invasive margin in pancreatic cancer is associated with tumor budding
Langenbeck's Archives of Surgery (2024)
-
Tumor budding and the prognosis of patients with metastatic colorectal cancer: a meta-analysis
International Journal of Colorectal Disease (2023)
-
Tumor Budding beim kolorektalen Karzinom – Informationen zur klinischen Anwendung und Anleitung zur praktischen Bestimmung
Der Pathologe (2022)