Abstract
Parkinson's disease is one of the most common conditions affecting the older generation. It is a progressive neurological condition presenting with motor, non-motor and behavioural symptoms that may impact upon oral health. Protecting and maintaining oral health for these individuals is paramount to their comfort, function and quality of life. To do so requires an individualised care plan considering the current stage and progression of their condition, access to care, ability to safely tolerate treatment and oral prostheses, their supporting network, and their own wishes. This paper reviews the current literature on Parkinson's disease and discusses two case reports of patients at differing stages of the condition. It emphasises the importance of a multidisciplinary approach, reasonable adjustments and the use of various techniques and skills within a dentist's armamentarium.
Similar content being viewed by others
Key points
-
Provides up to date knowledge about Parkinson's disease, an increasingly prevalent condition within the UK population.
-
Discusses the holistic dental management of two patients with Parkinson's disease.
-
Details the oral conditions patients with Parkinson's disease may face and potential strategies/solutions to manage them.
-
Details the potential complexities associated with dental treatment provision in Parkinson's disease and potential solutions to overcome them.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative condition after Alzheimer's disease,1 with a 2015 UK prevalence of approximately 137,000 cases.2 Age significantly increases risk, with diagnosis predominantly occurring after age 50 and peaking at 75-84.2,3 Rarely cases present before 40, being termed early onset PD.3 Males are at increased risk, being affected 1.5 times more frequently than females.2
PD occurs from the loss of dopamine producing neurons within the brain.3 Dopamine is a multifunctional neurotransmitter, working via four main pathways.4 Its gradual loss means these pathways alter or eventually cease, producing numerous symptoms which may impact oral health care and treatment. These are detailed in Table 1.
With an increasingly ageing population, there is a predicted 18% increase in PD between 2018 and 2025.2 Dental teams can thus expect to see and treat more of these patients. Current research suggests little or no experience among dental students in managing these groups;5 an issue that may negatively impact upon future practising patterns.
This paper aims to provide an update on PD knowledge and discusses the holistic management of two patients with the condition. It considers the oral conditions these individuals may face and treatment strategies to manage them. While specialist care may be necessary at more advanced stages, it highlights simple modifications and techniques the general dental practitioner (GDP) may use to help deliver the care these individuals need.
Current thinking around PD
Aetiology and pathophysiology
PD is predominantly considered idiopathic with both genetic and environmental aetiologies.3 A meta-analysis in 2012 reported twelve specific high-risk genes,6 while environmental factors have included infective agents, toxic agents, emotional stress and traumatic head injuries.7,8 While the exact mechanism for neuronal loss is unknown, cellular mitochondrial dysfunction is advocated, resulting in loss of cellular repair mechanisms and the production of large quantities of free radicals/oxidative stress.3,9 Aggregates of misfolded proteins (Lewy bodies), are isolated within the brains of PD sufferers. These are largely considered the by-product of these dysfunctional cell systems and once built up to harmful levels, trigger cell death.3,10
These changes activate the brain's immune cells (microglia), producing an inflammatory reaction which contributes to further neurotoxicity.11,12 With increasing evidence of biological links between periodontal disease and other inflammatory disorders, a link with PD is also postulated, with the inflammatory by-products of periodontal disease potentially initiating or exacerbating the neurodegeneration within the brain.13
Treatment
Currently, there is no cure for Parkinson's disease. Treatments aim to reduce symptoms, delay progression and maintain quality of life. While predominantly pharmacological, non-pharmacological and surgical options may be considered depending on individual symptoms, condition severity and progression, drug-related adverse effects, clinician and patient experience, and economic constraints.14
Pharmacological treatments
Pharmacological treatment primarily aims to enhance brain dopamine. As dopamine itself cannot be given due to its inability to cross the blood-brain barrier, alternative options must be used. Dopamine's precursor (L-dopa) is the first line drug for treating motor disturbances,15 however longer term therapy results in motor complications, including dyskinesias (abnormal uncontrolled voluntary movements) and 'on and off' periods (fluctuations between moving with ease and moving with great difficulty).16 Attempts to control these effects are achieved by adding other dopamine formulations, to allow L-dopa doses to be reduced. These include dopamine agonists, monoamine oxidase B inhibitors and COMT inhibitors, which either mimic or enhance dopamine's effects. Antivirals are a newer drug class in PD treatment for advanced disease.16 Table 2 shows examples of each drug type.
Surgical treatment
Stem cell replacement therapy
As PD results from the degeneration of dopaminergic neurons, stem cell therapy to replace these neurons has been considered. This is currently in the experimental phase.17
Ablative surgery and deep brain stimulation
In advanced Parkinson's, surgical destruction or electrical stimulation of certain brain regions may correct the neurochemical imbalance present.18 While potentially effective, procedures carry the risk of stroke, confusion, worsening speech and vision, and are therefore reserved for patients with severe motor complications despite optimal pharmacotherapy.14,18
Non-pharmacological treatment
Options currently being researched but with limited conclusive evidence include acupuncture,14 physical therapies,14 and natural antioxidants such as vitamin E, coenzyme Q10, creatine, melatonin, omega-3 fatty acids and B vitamins.19,20
Smoking and PD
Cigarette smoking is shown to be a protective factor for PD development, though the mechanism is unclear.21 Current research focuses on investigating drugs using substances derived from tobacco and tobacco smoke.21
Case reports
Case A
A 68-year-old male attended the community dental clinic complaining of a gap at the front of his mouth, and sharp teeth on the left side. He had been diagnosed with PD in 2000 and suffered shaking and tremors daily, difficulty sleeping with daytime drowsiness, speech changes and a feeling of imbalance when walking. He was medicated with co-careldopa (eight/day), ropinirole TDS, rasagline OD, and apomorphine and zopiclone PRN. He required apomorphine up to four times daily as a rescue treatment during 'off periods'.
Previous dental treatment had been completed greater than 15 years ago with local anaesthesia at his general dentist. He had always attended regularly and cared about his appearance. Since his diagnosis, he had found access to care challenging and felt, due to his medical history and communication difficulties, dentists were disinterested in him. He lived with his wife and two sons and brushed his teeth twice daily using a whitening toothpaste and manual toothbrush. Dietary analysis revealed a highly cariogenic diet with an increased craving for sweet foods since his PD diagnosis.
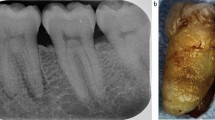
Extraoral examination was normal, however the patient exhibited sporadic involuntary jaw movements and an occasional delay on mouth opening. There was generalised plaque and calculus, gingival inflammation and toothpaste remains on the patient's teeth. His mouth was slightly dry with thicker saliva and a BPE of two in all quadrants. Clinical and radiographic examination revealed a very heavily restored dentition. Caries was present in the 34 and 35, a carious uncomplicated fracture of the 21, and a retained 22 root. Additionally, multiple worn anterior restorations and evidence of generalised tooth wear was present. The patient's preoperative radiographs are shown in Figure 1.
At the initial assessment, a sensitive discussion was held with the patient with regards to the progressive nature of PD and its potential impact on oral health. Numerous preventative therapies were implemented, including high fluoride (5,000 ppm) toothpaste, sodium fluoride mouthwash and saliva replacement spray. Treatment was staged over 12 appointments. During the stabilisation phase, preventative advice was reinforced at each visit and simple composite restorations completed on 11, 12, 21, 31, 32, 33, 41, 42 and 43. The 34 exhibited a frank pulpal exposure and was extirpated. The 35 was restored with biodentine/composite. The 22 was extracted and replaced with an immediate denture which the patient was unable to tolerate and had difficulties inserting and removing. By the sixth appointment, a review of risk status revealed significant improvements in oral hygiene and diet, with the patient brushing three times daily (once assisted) and reducing sugar intake.
The definitive treatment phase included a root canal treatment and crown on the 34, a conventional cantilever bridge from 21-22, and the construction of an upper biteguard; IV midazolam sedation was used to control movements for the longer root canal treatment and crown and bridge preparation appointments. The patient's clinical photographs at appointment two and post-treatment are shown in Figure 2.
Case B
A 63-year-old female attended the tertiary care clinic, complaining of a lost crown and dull pain from the lower left quadrant. She had been diagnosed with PD in 2005 and suffered symptoms of advanced disease, including Parkinson's dementia, severe dyskinesia, unsteady gait and a history of recurrent falls. She used a wheelchair when out of the house. Additionally, she had an overactive bladder, low blood pressure, anxiety and depression. She was medicated with co-careldopa (six/day), plus PRN, co-beneldopa BD, sertraline OD, clonazepam OD, rivastigmine transdermal patch OD, quetiapine OD, melatonin OD, adcal BD and alendronic acid.
The patient had been a regular dental attender until her Parkinson's symptoms limited her access to routine care. She had been referred by the community dental service who were unable to offer her treatment due to the severity of her dyskinesia. She lived in her own home with around the clock care and required help with most aspects of daily living. She had two adult children, with her daughter acting as her lasting power of attorney for health and welfare and finances.
The patient could transfer with support from wheelchair to dental chair. Extraoral examination was normal, however generalised, severe dyskinetic movements prevented an adequate intraoral examination or intraoral radiographs. Obviously carious/fractured teeth were noted and lateral oblique radiographs taken (Fig. 3), indicating a heavily restored dentition. Caries was noted on the 25, 27, 35, 36, 37, 46, and potentially the 47.
After discussion, treatment was planned under intravenous midazolam sedation to reduce her movements. The first appointment was for a full examination, to ascertain the prognosis of her remaining teeth along with a periodontal and soft tissue assessment. The patient was able to consent to this.
On the first sedation treatment, cannulation was achieved with minimal clinical holding. Midazolam was titrated slowly due to her relative frailty; 1 mg initial dose and 0.5 mg increments. Her movements stopped after 2 mg but quickly returned. Further 0.5 mg increments were titrated throughout the appointment to a total of 4 mg. She was placed on supplementary oxygen at 2 L per minute in response to her oxygen saturation levels dropping below 90% repeatedly. Treatment was abandoned due to these repeated drops in oxygen saturation, meaning that further midazolam could not be safely administered. Additionally, even when dyskinesias were reduced, the patient found it difficult to tolerate dental treatment. It was clear that she had significant treatment needs that could not be safely completed with sedation. General anaesthesia was therefore chosen to complete her treatment and a follow-up arranged to plan this.
At follow-up, there had been further progression of her Parkinson's. She had been admitted as a neurology inpatient for two weeks due to an exacerbation of dyskinesia, confusion and an inability to sleep. There had been further cognitive decline and she lacked capacity to consent to the proposed treatment. A best interests decision was made with her daughter for completion of treatment under day case general anaesthesia (GA), to include full clinical and radiographic examination, and any necessary dental care, including periodontal and restorative treatment, and the extraction of poor prognosis teeth.
Discussion
Treatment planning
Patients with PD may present to the dental team at varying stages of severity. While not clear cut, five main stages have been defined:22,23
- 1.
Stage one: mild symptoms, on one side of the body, with minimal or no functional impairment
- 2.
Stage two: symptoms affecting both sides of the body with daily tasks becoming more difficult
- 3.
Stage three: mid-stage with person remaining independent, but at increased risk of falls. Activities such as dressing and eating may become impaired
- 4.
Stage four: severe and limiting symptoms, with disabling instability while standing or walking; person requires substantial help and cannot live alone
- 5.
Stage five: advanced and debilitating symptoms, requiring wheelchair use and constant care.
Progression between stages varies significantly between individuals and is difficult to predict.24 While patients may wish for more complex treatments while independent, the longer term difficulties they may face with condition progression must be considered.
In case A, the patient was stage three, showing slow deterioration over 17 years. He was still independent, accessing care by bus or taxi except on 'bad days', when he benefited from family support. Additionally, while able to dress and eat, increased time was taken for these activities. While his initial oral risk status was high, regular and appropriate patient and family education enabled improvement.
The decision to carry out indirect restorations on the 21 and 34 resulted from a consideration of the patient's concerns about his missing front tooth, his inability to manipulate a denture, his improvement in risk status and an excellent support network who understood the progressive nature of PD and the requirement for assistance. Additionally, treatment could be provided comfortably and safely with the use of LA with or without sedation where required. Treatment was designed to facilitate cleansability, with supragingival margins and a modified ridge lab bridge pontic design.
In case B, the patient presented at stage four but progressed to stage five by follow-up. At such a high dependency level, complex work was inappropriate due to the extreme difficulties in performing and remedying treatment and maintaining oral health. The only option for treatment was GA, before which the patient was referred for a detailed preoperative medical assessment for the older person (POPS), to assess their fitness for surgery. Risks included an 11.4% morbidity risk, with potential postoperative delirium and functional decline. Additionally, mortality risk was 0.5%. With such risks, a repeat procedure needed to be avoided and a lower threshold for extractions employed.
Seattle Care Pathway
The Seattle Care Pathway 2014 provides a guide to maintaining oral health in older adults with varying dependency levels.25 Access, current and future oral risk, palliative versus elective dental care, and the use of multidisciplinary teams are considered. Overarching treatment advice is given, with key concepts including the easy maintenance of restorations and prostheses, repair/replacement of strategically important teeth guided by the shortened dental arch principle, and the use of the atraumatic restorative technique at higher dependency levels when invasive treatment or GA may be too risky. The use of implants to achieve a shortened dental arch (SDA) may be considered.
Implants in PD
As shown in case A, conventional dentures are often poorly tolerated in PD.26,27,28 Compromised muscular control affects prosthesis stability and adaptation,27,29 while involuntary movements affect insertion, removal and construction.29 Although implant-retained prostheses have been considered for this group, factors such as bruxism, dyskinesias, poor oral hygiene and poor balance may all increase risk of failure.27 Additionally, treatment provision may be unsafe due to involuntary movements, increasing the aspiration risk of small implant components, particularly in dysphagic patients.28,30
Despite this, short-term results in cases up to 12 months have been successful, with maintenance of implant stability and improvements in the patient's masticatory ability, prosthesis satisfaction and quality of life.29,31,32 Additionally, insertion and removal were not particularly problematic.29,32 Longer term analysis is, however, less promising. Packer et al., in a study of 12 patients, provided implants to both completely and partially edentulous adults with PD.32 At 12 months, three patients had died with the remaining nine showing maxillary success rates comparable to those in non-Parkinson's populations (around 85%), and mandibular rates to be lower (81% vs 95%). At seven years, only four patients were reviewed. All experienced prosthetic complications, mainly from dyskinetic parafunctional activity, leading to fracture of overdentures.28 While quality of life was still maintained, the cost and time of these complications was significant, averaging four appointments yearly.28 The long-term affordability of this treatment option, not routinely provided on the NHS, must also be considered.
Prevention
The heavily restored dentition presented in both cases is representative of many older patients, with 'natural' dentitions being retained for longer.33 While this has benefits, the risk of failing dentistry and pathology significantly rises. When combined with the other risk factors in PD, rates of caries and periodontal disease accelerate, being much greater than age-matched controls,34,35 and often increasing in direct proportion to PD severity.36
Motor impairments, cognitive and behavioural disturbances, and salivary alterations may all compromise oral hygiene,34 while stress and depression may directly increase periodontal risk, compromising the individual's host response.37 Reduced mastication and swallowing may encourage food retention, which when combined with the sugar cravings commonly seen in PD, increases caries risk dramatically.
In case A, oral hygiene was hampered by bradykinesia and dyskinesias of the arms and hands. A personalised care plan was developed, suggesting twice daily toothbrushing 30 minutes after medication when motor disturbances were reduced, and to switch to an electric toothbrush to reduce the need for patient manipulation. Honest discussions regarding the need for assisted brushing was emphasised and a bedi wedge suggested to enhance caregiver control during orofacial involuntary movements (Fig. 4).
Topical high fluoride therapies and dietary advice were provided. Altering the patient's medication to avoid xerostomia was considered, however both GP and patient were reluctant to change regime due to the considerable time it had taken to optimise symptomatic control.
Communication and capacity
Up to 90% of PD sufferers experience speech disturbances (dysarthria) during their condition.38 As facial expressions and hand gestures may also become compromised, non-verbal communication additionally suffers. In case A, the patient demonstrated fast, quiet, mumbling speech. He felt frustrated and embarrassed as healthcare professionals often ignored or spoke over him, questioning his ability to consent and talking to his wife instead.
To improve communication and empower the patient, simple strategies were adopted (Box 1).39,40 Additionally, a referral to speech and language therapy (SaLT) was initiated, a management strategy advocated by NICE.15 A six-month course of 'LOUD' and pacing therapy to increase speech volume and clarity was completed, with a notable difference observed by the seventh appointment. This not only aided communication during appointments but assisted in other aspects of life.
In case B, communication was hindered by the dementia diagnosis. In line with the Mental Capacity Act 2005, the patient initially had capacity to consent. However, this was deemed to be lacking at follow-up and during her POPS assessment. A best interests decision was therefore made with the patient's legal POA (her daughter in this case).
Controlling involuntary movements
Both patients suffered dyskinesias. In case A, the patient experienced 'on' and 'off' periods with off periods predominantly occurring towards the end of the three-hour co-careldopa period. Conversely, symptomatic relief was seen 30-60 minutes post-medication. Appointments were thus planned accordingly. For shorter, less complex treatment, this was satisfactory. However, for longer treatments, a small dose of IV midazolam sedation helped to reduce dyskinesias and facilitate treatment comfortably and safely. In case B, dyskinesias were continuous and unrelenting, despite medication. While IV midazolam reduced movements for a short while, this was insufficient for successful treatment, making GA the only option.
Potential side effects of Parkinsonian medications may include sleep attacks, drowsiness, sedation and blood pressure disturbances.16,41 These should be considered when using conscious sedation or GA. Additionally, recognising the older age of most Parkinson's sufferers, a slower titration regime (as was employed in case B) avoids the risk of over sedation and respiratory depression.
Bruxism and PD
Patient A was aware of daytime clenching and grinding, a finding commonly reported in PD, and contributing to an increased incidence of temporomandibular disorders, tooth wear and fractures.42 A mouthguard was suggested in the initial instance, with a notch in the upper border to aid removal. Other successful management strategies include botox, reported to decrease secondary bruxism for up to 1-5 months with improvement in pain and mandibular functions.43
Dysphagia
Dysphagia (an impaired swallow) commonly follows dysarthria, and affects 40-95% of PD sufferers.38 This may present significant risks during dental treatment, increasing the possibility of oral bacterial aspiration and aspiration pneumonia, currently the most common cause of death in PD.44 Identification of 'at risk' patients is crucial and should be explored during initial medical history-taking. Patients may present with signs and symptoms (Box 2)45 suggestive of dysphagia and an onward referral to SaLT should be considered.
Recommended modifications to oral health care and dental treatment are summarised in Table 3.44 For those at the highest risk, the need for elective treatment must be thoroughly considered and, if required, referral made to specialist services.
Conclusion
Parkinson's disease is an increasingly prevalent condition, presenting numerous symptoms that may impact upon oral health and the ability to provide dental treatment. Holistic management embraces many issues common to special care patients, including patient communication, cooperation, education, prevention, access and consent. When treatment planning individuals with progressive conditions, a personalised, multidisciplinary approach is essential. While many patients with PD can be safely treated in general dental practice, referral to specialised services may be necessary at more advanced stages. Developments in medical and dental treatments, together with increased life expectancy, may result in implants being used more commonly in the future, however this requires further research.
References
Lebouvier T, Chaumette T, Pailusson S et al. The second brain and Parkinson's disease. Eur J Neurosci 2009; 30: 735-741.
Parkinson's UK. The Incidence and Prevalence of Parkinson's in the UK. 2017. Available at https://www.parkinsons.org.uk/professionals/resources/incidenceandprevalenceparkinsonsuk-report (accessed June 2019).
World Health Organisation. Neurological Disorders: Public Health Challenges. 2006. Available at http://www.who.int/mental_health/neurology/neurological_disorders_report_web.pdf (accessed June 2019).
Ayano G. Dopamine: Receptors, Functions, Synthesis, Pathways, Locations and Mental Disorders: Review of Literatures. J Ment Disord Treat 2016; 2: 120.
Jeter C B, Rozas N S, Sadowsky J M, Jones D J. Parkinson's Disease Oral Health Module: Interprofessional Coordination of Care. MedEdPORTAL 2018; 14: 10699.
Lill C M, Roehr J T, McQueen M B. Comprehensive research synopsis and systematic meta-analyses in Parkinson's disease genetics: The PDGene database. PLoS Genet 2012; 8: e1002548.
Tanner C M, Goldman S M. Epidemiology of Parkinson's disease. Neurol Clin 1996; 14: 317-335.
Jafari S, Etminan M, Aminzadeh F, Samii A. Head injury and risk of Parkinson disease: a systematic review and meta-analysis. Mov Disord 2013; 28: 1222-1229.
Schapira A H. Mitochondrial dysfunction in Parkinson's disease. Cell Death Differ 2007; 14: 1261-1266.
National Institute of Neurological Disorders and Stroke. Parkinson's Disease: Hope through Research. What causes the disease? Available at https://www.ninds.nih.gov/Disorders/PatientCaregiverEducation/HopeThroughResearch/ParkinsonsDiseaseHopeThroughResearch#causes (accessed June 2019).
Nagatsu T, Sawada M. Inflammatory process in Parkinson's disease: role for cytokines. Curr Pharm Des 2005; 11: 999-1016.
Tansey M, Sulzer D, Standaert D G. Inflammation in Parkinson's disease. 2016. Available at https://www.movementdisorders.org/MDS/ScientificIssuesCommittee-Blog/InflammationinPD.htm (accessed June 2019).
Kaur T, Uppoor A, Naik D. Parkinson's disease and periodontitis the missing link? A review. Gerodontology 2016; 33: 434-438.
Chen J J. Parkinson disease: A summary of recent evidence-based medicine reviews. Mental Health Clinician 2012; 2: 25-31.
National Institute for Health and Care Excellence. Parkinson's disease in adults: pharmacological management of motor symptoms. 2017. Available at https://www.nice.org.uk/guidance/ng71/chapter/Recommendations#pharmacologicalmanagementofmotorsymptoms (accessed June 2019).
Munchau A, Bhatia K P. Pharmacological Treatment of Parkinson's Disease. BMJ 2000; 76: 602-610.
Barker R A. The Trials and Tribulation of Cell Transplants for Parkinson's Disease! 2017. Available at https://www.worldpdcongress.org/home/2017/3/24/thetrialsandtribulationofcelltransplantsforparkinsons-disease (accessed June 2019).
National Institute for Health and Care Excellence. Interventional procedure overview of subthalamotomy for Parkinson's disease. 2004. Available at https://www.nice.org.uk/guidance/ipg65/documents/overviewofsubthalamotomyforparkinsons-disease2 (accessed June 2019).
Filograna R, Beltramini M, Bubacco L, Bisaglia M. Anti-Oxidants in Parkinson's Disease Therapy: A Critical Point of View. Curr Neuropharmacol 2016; 14: 260-271.
Mischley L K, Lau R C, Bennett R D. Role of Diet and Nutritional Supplements in Parkinson's Disease Progression. Oxid Med Cell Longev 2017; 6405278. DOI: 10.1155/2017/6405278.
Li X, Li W, Liu G, Shen X, Tang Y. Association between cigarette smoking and Parkinson's disease: A meta-analysis. Arch Gerontol Geriatr 2015; 61: 510-516.
Parkinson's Foundation. Stages of Parkinson's. Available at https://parkinson.org/Understanding-Parkinsons/WhatisParkinsons/StagesofParkinsons (accessed June 2019).
Hoehn M M, Yahr M D. Parkinsonism: onset, progression, and mortality. Neurology 1967; 17: 427-442.
Schrag A, Dodel R, Spottke A, Bornschein B, Siebert U, Quinn N P. Rate of clinical progression in parkinson's disease. a prospective study. Mov Disord 2007; 22: 938-945.
Pretty I A, Ellwood R P, Lo E C et al. The Seattle Care Pathway for securing oral health in older patients. Gerodontology 2014; 31 (Spec Iss): 77-87.
Fiske J, Hyland K. Parkinson's disease and oral care. Dent Update 2000; 27: 58-65.
Romero-Perez M J, Mang-de la Rosa Mdel R, Lopez-Jimenez J, Fernandes-Feijoo J, Curando-Soriano A. Implants in disabled patients: a review and update. Med Oral Patol Cir Bucal 2014; 19: e478-e482.
Packer M E. Are dental implants the answer to tooth loss in patients with Parkinson's disease? Prim Dent J 2015; 4: 35-41.
Heckmann S M, Heckmann J G, Weber H P. Clinical outcomes of three Parkinson's disease patients treated with mandibular implant overdentures. Clin Oral Implan Res 2000; 11: 566-571.
Deliberador T M, Marengo G, Scaratti R, Giovanini A F, Zielak J C, Baratto Filho F. Accidental aspiration in a patient with Parkinson's disease during implant-supported prostheses construction: A case report. Spec Care Dentist 2011; 31: 156-161.
Chu F C, Deng F L, Siu A S, Chow T W. Implant-tissue supported, magnet-retained mandibular overdenture for an edentulous patient with Parkinson's disease: a clinical report. J Prosthet Dent 2004; 91: 219-222.
Packer M, Nikitin V, Coward T, David D M, Fiske J. The potential benefits of dental implants on the oral health quality of life of people with Parkinson's disease. Gerodontology 2009; 26: 11-18.
Steele J G, Treasure E T, O'Sullivan I, Morris J, Murray J J. Adult Dental Health Survey 2009: transformations in British oral health 1968-2009. Br Dent J 2012; 213: 523-527.
Muller T, Palluch R, Jackowski J. Caries and periodontal disease in patients with Parkinson's Disease. Spec Care Dentist 2011; 31: 178-181.
Cicciu M, Risitano G, Lo Giudice G, Bramanti E. Periodontal health and caries prevalence evaluation in patients affected by Parkinson's disease. Parkinsons Dis 2012; 541908. DOI: 10.1155/2012/541908.
Hanaoka A, Kashihara K. Increased frequency of caries, periodontal disease and tooth loss in patients with Parkinson's disease. J Clin Neurosci 2009; 16: 1279-1282.
Wimmer G. Janda M, Wieselmann-Penkner K, Jakse N, Polansky R, Pertl C. Coping with stress: its influence on periodontal disease. J Periodontol 2002; 73: 1343-1351.
Tjaden K. Speech and Swallowing in Parkinson's Disease. Top Geriatr Rehabil 2008; 24: 115-126.
British Society of Gerodontology. Guidelines for the Oral Healthcare of Stroke Survivors. 2010. Available at https://www.gerodontology.com/content/uploads/2014/10/stroke_guidelines.pdf (accessed June 2019).
American Speech-Language-Hearing Association. Dysarthria: Tips for Talking with Someone Who Has Dysarthria. Available at https://www.asha.org/public/speech/disorders/dysarthria/#tips (accessed June 2019).
National Institute of Neurological Disorders and Stroke. Parkinson's Disease: Hope Through Research: How is the disease treated? Available at https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Parkinsons-Disease-Hope-Through-Research#Treatment (accessed June 2019).
Verhoeff M C, Lobbezoo F, Wetselaar P, Aarab G, Koutris M. Parkinson's disease, temporomandibular disorders and bruxism: A pilot study. J Oral Rehabil 2018; 45: 854-863.
Guaita M, Hogl B. Current treatments of bruxism. Curr Treat Options Neurol 2016; 18: 10.
All Wales Special Interest Group. Dysphagia and Oral Health: Recommendations for the Dental Team for the Management of Oral Healthcare of Children and Adults with Dysphagia. 2014. Available at http://www.sigwales.org/wp-content/uploads/sig-dysphagia-guidelines1.pdf (accessed June 2019).
World Gastroenterology Organisation. Dysphagia: Global Guidelines & Cascades. 2014. Available at http://www.worldgastroenterology.org/UserFiles/file/guidelines/dysphagiaenglish2014.pdf (accessed June 2019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaka, S., Lane, H. & Sherwin, E. Dentistry and Parkinson's disease: learnings from two case reports. Br Dent J 227, 30–36 (2019). https://doi.org/10.1038/s41415-019-0470-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-019-0470-9
This article is cited by
-
Oral health experiences of people living with Parkinson's disease: a scoping review
British Dental Journal (2024)
-
Prostheses in Parkinson's disease
British Dental Journal (2019)