Abstract
Exosomes participate in many physiological and pathological processes by regulating cell–cell communication, which are involved in numerous diseases, including osteoarthritis (OA). Exosomes are detectable in the human articular cavity and were observed to change with OA progression. Several joint cells, including chondrocytes, synovial fibroblasts, osteoblasts, and tenocytes, can produce and secrete exosomes that influence the biological effects of targeted cells. In addition, exosomes from stem cells can protect the OA joint from damage by promoting cartilage repair, inhibiting synovitis, and mediating subchondral bone remodeling. This review summarizes the roles and therapeutic potential of exosomes in OA and discusses the perspectives and challenges related to exosome-based treatment for OA patients in the future.
Similar content being viewed by others
Introduction
Osteoarthritis (OA) is a highly prevalent type of degenerative joint disease that affects over 300 million people worldwide.1 Chronic pain and motion dysfunction induced by OA seriously reduced the quality of life of patients. In addition, the socioeconomic burden of OA on patients and society is considerable. Current OA management is broadly divided into nonpharmacological, pharmacological, and surgical treatments.2,3,4 Nonpharmacological treatments, such as exercise, weight loss, and physical therapy, are suggested as the appropriate therapy for early-stage OA patients. Pharmacological treatments are mainly aimed at achieving pain control for better function and quality of daily life. Surgical treatment is most widely used for end-stage patients with serious functional disability. At present, there are few satisfactory strategies to improve joint homeostasis and delay OA progression.3,5 Understanding the underlying mechanisms of OA can facilitate the development of novel therapies for future clinical needs.
OA has been previously described primarily in terms of articular cartilage destruction, but accumulating evidence has revealed that OA is a disease with whole-joint damage and dysfunction.6,7 During OA progression, the pathologic changes in joints include cartilage damage, remodeling of the subchondral bone, inflammatory activation in the synovium, degeneration of ligaments and the menisci, and changes in the joint capsule, bursa, periarticular muscles, nerves, and local fat pads. Several factors have been revealed to be associated with pathological changes in the OA joint, including aging, trauma, mechanical loading, and genetic and metabolic disorders.4,8 Moreover, the different tissues in the joint could influence each other during the course of OA, which may synergistically contribute to OA pathology and clinical symptoms.9,10,11 Subchondral bone is a layer of cortical bone below the articular cartilage and the underlying trabecular bone in the joint, which was recently proposed to play a significant role in OA pathogenesis. The subchondral bone could affect cartilage degeneration through mechanical changes or paracrine-mediated bone-cartilage cross-talk.12,13,14 The cytokines from synovial fibroblasts (SFB) of inflammatory cells could influence the degradation of the cartilage matrix and the formation of osteophytes by releasing proinflammatory factors such as IL-1β and bone-regulated factors including BMP-2.15 Inflammatory activation of the synovium and infrapatellar fat pad (IPFP) can lead to the release of various proinflammatory mediators that not only cause widespread changes in the structure and function of synovial tissue but also promote articular cartilage damage and accelerate OA development.15,16,17 Therefore, investigating intercellular communication within and/or among different joint cells during OA development could be beneficial for understanding the pathogenesis of OA and exploring new therapeutic strategies for OA in the future.
Exosomes are considered important mediators of cell–cell communication that participate in numerous physiological and pathological processes. Recently, the roles and therapeutic potential of exosomes in OA have been increasingly addressed in this field. In this review, we summarize the existing research on exosomes in OA and discuss the perspective and challenges related to exosome-based treatment for OA patients.
Exosome
Intercellular communication mediator
Extracellular vesicles (EVs) are membrane-bound vehicles that can be divided into three types, including exosomes, microvesicles (MVs), and apoptotic bodies.18 As an important kind of EV, exosomes have received the most attention over the past decade. Exosomes can be secreted by various cells and mediate intercellular communication via their contents, including lipids, nucleic acids, and proteins.
The diameter of exosomes usually ranges from 30–150 nm, and the density is between 1.13 and 1.19 g·mL−1.19 Trams et al. found that exfoliated membrane vesicles may serve a physiologic function and suggested these vesicles as exosomes.20 In 1983, Harding et al. observed that membrane-bound vesicles could be released by multivesicular endosome (MVE) exocytosis.21 Later, researchers found that the transferrin receptor could transfer from the surface of the cell into internal vesicles to form MVEs.22 In 1987, Johnstone et al. observed that exosome release during reticulocyte maturation was associated with plasma membrane activities.23 Raposo et al. later found that exosomes played an important role in antigen presentation and T cell activation.24 Then, the relationship between exosomes and tumors was reported.25,26 In 2007, Valadi et al. found that mRNA and microRNA can be sent to other cells by exosomes, indicating that exosomes may mediate intercellular communication by delivering nucleic acids.27 Thereafter, an increasing number of studies have shown that exosomes play important physiological and pathological roles by mediating cell–cell communication.28,29
Exosome biogenesis
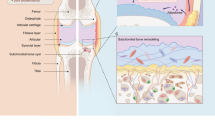
Exosome biogenesis can be divided into different phases, including early endosome formation by invagination of the plasma membrane, late endosome formation by cargo selection, the formation of multivesicular bodies (MVBs) from late endosomes and membrane fusion between MVBs and the plasma membrane, leading to the release of the vesicular contents, named exosomes30,31,32 (Fig. 1). The endosomal sorting complex required for transport (ESCRT), gene-2-interacting protein X linked by apoptosis and tumor susceptibility gene 101 (TSG-101), is mainly responsible for the formation and release of exosomes.33,34,35,36 The ESCRT is mainly composed of ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III in complex.37 ESCRT-0, -I and -II contain ubiquitin-binding subunits that can capture ubiquitin-tagged cargo, while ESCRT-III contributes to vesicle budding and scission.38,39 However, exosome formation can occur independently of the ESCRT complex under certain conditions. The lipids are capable of promoting exosome formation. For example, the transfer of exosome-associated domains into the endosome lumen in a ceramide-activated model does not rely on ESCRT function.39,40
Biogenesis of exosomes. a Exosomes contain different types of proteins, nucleic acids, amino acids, and metabolites, in which CD9, CD63, CD81, flotillin, and Annexins could be used as markers. b Extracellular constituents along with cell surface proteins enter cells via the manners of endocytosis and plasma membrane invagination. Plasma membrane bud formation in the luminal side and the fusion of the bud with the constituents of the endoplasmic reticulum (ER), trans-Golgi network (TGN), and mitochondria lead to the formation of early sorting endosomes (ESEs). Then, ESEs give rise to late sorting endosomes (LSEs) in which second invagination via modification of the cargo, leading to the generation of various intraluminal vesicles (ILVs) and the formation of multivesicular body (MVBs). Next, some of MVBs fuse with lysosomes, and the contents in MVBs undergo degradation. Other MVBs can be transported to the plasma membrane and dock on the luminal side of cells. Finally, the exocytosis of MVBs releases ILVs as exosomes to the outside of cells. c Exosomes enter cells by different manners including fusion with cell plasma membranes, receptor-mediated entry, clathrin-coated pits, lipid rafts and so on
Methods of exosome separation
The enrichment of exosomes with high purity was the critical step for basic research and the further clinical application of exosomes. Currently, different methods for exosome separation, including ultracentrifugation, size-based techniques, immunoaffinity purification, precipitation, and microfluidics-based isolation techniques, have been developed based on the size, shape, density, and surface proteins of exosomes. These methods have their own advantages and disadvantages and need to be further improved to promote the research on exosomes and their application.41,42,43,44,45
Ultracentrifugation
Ultracentrifugation is the most common method to separate different biological components, such as viruses, bacteria, subcellular organelles, and EVs.43 Ultracentrifugation is currently considered the classical method of exosome isolation and can be divided into density gradient ultracentrifugation and differential ultracentrifugation. A centrifugal force between ×100 000 and 150 000 g is often used in this separation method. However, ultracentrifugation has some shortcomings, such as requiring advanced supercentrifugation and consuming extensive time. Moreover, ultracentrifugation may influence the structures of exosomes, which would impede downstream analysis.
Size-based technique
Size-based technique such as ultrafiltration is another kind of isolation method and has been used to harvest exosomes from urine, serum, cerebrospinal fluid, and cell culture medium by particle size or molecular weight.42,46,47 This exosome isolation method does not require complex equipment, and the operation procedure is relatively simple. However, this method still poses some challenges.42,43 For example, the isolated exosome may be contaminated by molecular aggregates, decreasing exosome purity. In addition, the shear stress produced in the isolation process may induce the deterioration of exosomes.
Immunoaffinity purification
Based on marker proteins in the bilayer membranes of exosomes, exosomes can be selectively separated through immunoprecipitation mediated by specific immobilized antibodies.42 This isolation method could effectively improve exosome purity in some cases without complicated procedures. In addition, immunoaffinity purification could distinguish different subgroups of exosomes according to their special marker proteins, which may be beneficial for detailed mechanistic investigations in the future. Nevertheless, this method requires excellent antibody specificity, rigorous sample preparation, and costly reagents. In addition, only those EVs expressing the antibody-recognized protein can be separated, which may result in a low yield of exosomes.48
Precipitation
Precipitation with polyethylene glycols (PEGs) is widely used to isolate viruses and small particles.43,49,50,51 Polymers can adhere to water molecules, decreasing exosome solubility, and this effect can be used to separate exosomes from conditioned media, serum, or urine.48 The use of precipitation for exosome isolation is convenient and does not require special equipment. In addition, the concentration of isolated exosomes is relatively high. However, there are still many problems with this method, such as low recovery and high impurities.
Microfluidics-based isolation techniques
Microfluidics-based isolation is an alternative isolation method that is based on physical and biochemical properties, such as size, density, and immune affinity. In addition, it is a new sorting method that involves acoustic, electrophoretic, and electromagnetic procedures.52,53,54 The steps of this method mainly include immunoaffinity, sieving, and trapping exosomes on porous structures.55 This method consumes small amounts of sample volume, reagents, and separation time.56 In addition, microfluidics-based isolation could synergistically enrich exosomes and improve purity in combination with other exosome separation methods.43 However, this method requires advanced equipment, which may restrict its large-scale application.
Exosome identification
Exosome identification is mainly based on morphological features, particle size, and signature proteins such as CD9, CD63, CD81, and HSP90.57 There are different methods to identify the characteristics of exosomes.43 First, scanning electron microscopy (SEM) or transmission electron microscopy (TEM) can be used to identify exosomes directly. SEM observes the exosome surface microstructure, while TEM has a maximal resolution of 0.2 nm and can reveal the internal structure and morphology of exosomes.58 Second, nanoparticle tracking analysis (NTA) could analyze the particle size and the concentration of exosomes. The process of NAT-based detection is relatively simple, and the result can be better quantified. Third, western blot technology contributes to estimating specific marker proteins in exosomes, including CD63, CD8, TSG101, flotillin-1, ALIX, CD9, CD81, and CD82. Fourth, flow cytometry (FCM) can be used to analyze the size of exosomes by labeling targeted exosomes with specific antibodies or fluorescent dyes. FCM has some advantages for exosome analysis, including high-throughput screening and data quantification. Moreover, FCM can be used to distinguish different subpopulations of exosomes. In addition to the above methods, atomic force microscopy, tunable resistive pulse sensing, and dynamic light scattering (DLS) could also be used for the identification of exosomes.43
The function of exosomes in bone homeostasis
Over the past decades, exosomes have been found to affect several physiological and pathological processes via the exosomal contents, including RNAs, DNAs, proteins, and lipids.59 To date, cumulative evidence has revealed that exosomes participate in many biological processes, including angiogenesis, cell differentiation, immunomodulation, metabolic balance, and development,24,26,60,61,62 and are highly involved in many diseases, such as cancer, neurodegenerative disease, autoimmune diseases, and cardiovascular diseases.63,64,65,66,67,68,69,70,71,72,73 Recently, the roles of exosomes in bone homeostasis have been extensively addressed.73
Bone homeostasis is primarily maintained by bone resorption and bone formation, which are involved in various types of cells, including osteoblasts, osteoclasts, osteocytes, and MSCs.74 Abnormalities in bone homeostasis are closely related to bone diseases in several ways, such as affecting the onset and progression of osteoporosis and the wound repair of bone fractures. Many studies have shown that cells in the bone microenvironment can secrete exosomes to regulate bone resorption and bone formation. Osteoblast-derived exosomes carry potential osteogenesis-related signaling, such as the eukaryotic initiation factor-2 pathway, which may participate in bone formation.75 Exosomes from mineralizing osteoblasts could promote the differentiation of bone marrow stromal cells to osteoblasts.76 Exosomes from osteoclasts could inhibit osteoblast function, osteogenic differentiation, and bone formation via exosomal miRNAs, including miR-214-3p and miR-23a-5p.77,78,79 miR-218 contained in osteocyte-derived exosomes plays an important role in the myostatin-mediated inhibition of osteoblastic differentiation.80 In addition, MSCs can produce and release exosomes to participate in maintaining bone homeostasis. The exosomes produced by MSCs can prevent osteocytes from undergoing apoptosis in a hypoxia/serum deprivation model and glucocorticoid-induced osteonecrosis model.81,82 The exosomes excreted by iPS-derived MSCs can promote the regeneration of bone defects via enhanced angiogenesis and osteogenesis in an ovariectomized rat model.83 Exosomal transfer RNA-derived fragments from the plasma of osteoporosis patients, including tRF-25, tRF-38, and tRF-18, were significantly increased compared with those from healthy controls, suggesting that these tRFs may be new diagnostic biomarkers for osteoporosis.84 Exosomes from the BMSCs of osteoporosis patients decrease the osteogenic ability of MSCs by targeting the microRNA-21/SMAD7 pathway.85 Exosomes from different tissue-derived MSCs or iPS cell-derived MSCs could prevent bone loss, promote bone formation, and prevent osteoporosis in experimental animal models,86,87,88,89 indicating that MSC-derived exosomes could be a potential therapeutic treatment for osteoporosis.90
In addition to osteoporosis, MSC-derived exosomes also greatly contribute to fracture healing.91 The exosomes from hypoxically preconditioned MSCs had a higher miR-126 level and promoted fracture healing via the SPRED1/Ras/Erk signaling pathway.92 The injection of BMSC-derived exosomes can rescue the retardation of fracture healing in the femur fracture model of CD9(−/−) mice.93 Similarly, BMSC-derived exosomes can enhance osteogenesis and angiogenesis partially through the BMP-2/Smad1/RUNX2 and HIF-1alpha/VEGF pathways at fracture sites to accelerate fracture repair in a femoral nonunion rat model.94 Moreover, umbilical cord MSC-derived exosomes also promote fracture healing in a rat model of stabilized fracture, which is involved in the HIF-1alpha-mediated regulation of angiogenesis.95
In addition to osteoporosis and fracture, an increasing number of studies have revealed that exosomes are closely involved in OA pathology and have strong therapeutic potential for this disease.
Exosomes in OA
The roles and underlying mechanisms of exosomes in OA have attracted great interest from researchers. To date, there are two main research directions in this field. One set of studies have mainly focused on the diagnostic significance and biological effects of endogenous exosomes during OA (Fig. 2). In another, researchers have paid attention to the therapeutic effects of stem cell-derived exosomes on OA and potential optimization strategies (Fig. 3).
The exosomes from different tissues of OA joint and their potential biological effects. a The exosomes can be detected in the articular cavity and changed during OA progression. Some studies showed that the joint cells including chondrocytes, osteoblasts of subchondral bone, synovial mesenchymal stem cells (MSCs) and fibroblasts, infrapatellar fat pad MSCs, tenocytes and tendon stem cells as well as periodontal ligament cells and stem cells produce and release exosomes, which may be involved in the regulation of joint homeostasis. b The exosomes derived from different joint cells could mediate cell–cell communications and regulate diverse cell phenotype including cell proliferation, migration, differentiation, autophagy, matrix synthesis, inflammatory reaction and etc
Exosomes from joint tissues
Some research has indicated that exosomes are present in human synovial fluid (SF).96,97,98,99 Moreover, the quantity and composition of SF-derived exosomes could be changed in joint diseases, including OA, rheumatoid arthritis, and reactive arthritis.99,100,101 Skriner et al. reported that citrullinated proteins, including fibrinogen D fragment, Sp alpha (like CD5 antigen protein) receptor, fibrinogen beta-chain precursor, Sp alpha (like CD5 antigen protein) receptor, fibrin beta-chain, and fibrin alpha-chain fragment, were observed in synovial exosomes from RA patients but not in those with OA, indicating that exosomes carrying citrullinated peptides may be unique in different joint diseases.97 Kolhe et al. revealed the characterization of miRNAs in exosomes from the SF of OA patients. Their data showed that the SF-derived exosomal miRNAs differed between male and female OA patients and that the specific miRNAs for female OA patients are relevant to the estrogen response and toll-like receptor signal.99 OA SF-derived EVs functionally influence the expression of anabolic and catabolic activity-related genes.99 In addition, Domenis et al. found that exosomes from the SF of patients with gonarthrosis function to enhance the production of proinflammatory factors from M1 macrophages, which suggests that SF-derived exosomes may play a role in the pathological process of joint diseases.96
Zhao et al. reported that SF samples obtained from both early OA and late-stage OA contained much higher exosome levels than those from controls.101 In addition, the levels of exosomal lncRNA PCGEM1 from OA SF gradually increased with OA progression.101 Moreover, Gao, K. demonstrated that exosomes from end-stage knee OA patients have a higher level of chemokines and could recruit inflammatory cells and inhibit cartilage proliferation, which may ultimately promote joint degeneration.98 The above data provide the clinical relevance of exosomes and OA pathology, which improves our understanding of the OA microenvironment from the new perspective of exosomes (Table 1).
Cartilage-derived exosomes
Chondrocytes are the only cell type in articular cartilage. Previous studies have revealed that chondrocytes can release small membrane-bound extracellular articular cartilage matrix vesicles (ACVs) with a diameter of 100 nM to participate in the pathologic mineralization of osteoarthritic articular cartilage.102,103 Exosomes have been shown to share many features of matrix vesicles, including lipid and protein content, size and morphology, suggesting that matrix vesicles and exosomes may be homologous.104 Moreover, these ACVs contain significant amounts of RNA, similar to exosomes, which are protected from enzymatic degeneration and can be transferred to intact primary chondrocytes.105 The distinction between ACVs and chondrocyte-derived exosomes is still unclear and needs more research. Nevertheless, an increasing number of studies have revealed that both ACVs and chondrocyte-derived exosomes could mediate cell–cell communication.105,106,107,108,109 Exosomes from primary chondrocytes cultured in a normal environment (D0 exosomes) could restore mitochondrial dysfunction and enhance M2 macrophage penetration with a reduction in M1 macrophages.106 The intra-articular administration of D0 exosomes efficiently delayed OA progression.106 We found that exosome-like vesicles derived from osteoarthritic chondrocytes could stimulate inflammasome activation and increase mature IL-1β production in macrophages via the miR-449a-5p/ATG4B/autophagy pathway, which may aggravate synovitis and accelerate the progression of OA.107 In addition, Chen et al. reported that exosomes derived from chondrocytes (CC-Exos) could induce efficient ectopic chondrogenesis of cartilage progenitor cell (CPC) constructs in subcutaneous environments, which may represent a cell-free therapeutic approach for cartilage regeneration.108 Moreover, chondrocyte-secreted exosomes could affect chondrogenic differentiation and cartilage matrix synthesis via the miR-95-5p-regulated expression of HDAC2/8.109 The exosomes from OA chondrocytes could inhibit cell proliferation and enhance the apoptosis of chondrocytes, which was involved in the GSK-3β-mediated enrichment of HULC and decrease in miR-372-3p in exosomes.110 In addition, exosomes from chondrocytes could promote the chondrogenic differentiation of BMSCs by activating the Wnt/β-catenin pathway, which was related to the inhibition of GSK-3β expression by exosomal miR-8485.111 These results supported that chondrocytes could produce exosomes that mediate cell–cell communication, which play a functional role in AC maintenance and OA pathogenesis.
Synovium-derived exosomes
The synovium is a key component of synovial joints and greatly contributes to maintaining the homeostasis of articular cartilage. Abnormalities of the synovium, especially low-grade inflammation, have been reported to be associated with clinical signs and joint histopathology of OA patients.112,113,114,115,116,117 The inflammatory and anti-inflammatory cytokines produced by synovial macrophages and synoviocytes have been found to play important roles in regulating cartilage degradation and osteophyte formation.15,118,119,120 However, the role and cross-talk between synovium-derived exosomes and joint cells in OA pathology are poorly understood.
Headland et al. reported that neutrophil-derived MVs can penetrate cartilage, indicating that EVs from the synovium could mediate the cross-talk between the synovium and cartilage.100 Kato et al. isolated exosomes from IL-1beta-motivated SFB of human normal knee joints and cocultured these exosomes with articular chondrocytes.121 They found that exosomes from IL-1beta-stimulated SFB dramatically increased the levels of catabolism-related genes, including MMP-13 and ADAMTS-5, while decreasing the expression of anabolism-related genes, including COL2A1 and ACAN.121 Moreover, the exosomes from IL-1beta motivated SFBs to induce more proteoglycan derived from cartilage explants.121 These data first suggested that exosomes from the synovium could induce OA-like changes in vitro and ex vivo.121 In addition, synovial mesenchymal stem cells (MSCs) could also produce functional exosomes (SMSC-Exos) that could be taken up by articular chondrocytes.122 These SMSC-Exos activated YAP via dephosphorylation and decreased ECM secretion, and they induced articular chondrocyte proliferation and migration via Wnt5a and Wnt5b signaling.122 Moreover, exosomes collected from miR-140-5p-overexpressing synovial MSCs (SMSC-140s) promoted the proliferation and migration of articular chondrocytes but did not affect the secretion of ECM in vitro.122 The SMSC-140s also significantly ameliorated the severity of joint wear in a rat OA model.122 These findings revealed that the synovium could release functional exosomes, and their function can be regulated by their contents. In addition to SFB and MSCs, exosomes from other synovial cells, including macrophages, T cells and endothelial cells, should also be given close attention. Recently, Tsuno et al. investigated the effects of salazosulfapyridine and methotrexate on the proteome of exosomes produced by a human synovial sarcoma cell line (SW982).123 Their data indicated that these anti-rheumatic drugs changed the protein profiles of SW982-derived exosomes and inhibited the effect of IL-1beta on the exosomal proteome.123 The effects of anti-OA strategies on synovium-derived exosomes are worth further study.
Subchondral bone-derived exosomes
Subchondral bone plays an important role in the protection of normal joints and undergoes structural changes, including bone sclerosis, during the OA process.124,125 The remodeling of subchondral bone was strongly associated with the grade of cartilage lesions in clinical OA patients126 and somewhat related to joint pain in some cases.127 In experimental OA models, targeting specific signals in subchondral bone, such as TGF-β, could attenuate the pathological severity of OA and reduce the pain response.13,128,129,130 Previous studies revealed that exosomes could function by regulating the TGF-β signal of their targeted cells.131,132,133 In addition, Cai et al. reported that exosomes from TGF-β1 gene-modified dendritic cells delayed the development of inflammatory bowel disease by increasing CD4 + Foxp3 + Tregs and inhibiting Th17 in a dextran sodium sulfate (DSS)-induced mouse model.134 The effects of exosomes on the signaling pathway of subchondral bone cells, including TGF-β signaling, and their potential roles in OA progression deserve further attention. Cells, including osteoblasts, osteoclasts, osteocytes and bone marrow MSCs, can secrete exosomes to regulate the bone microenvironment and mediate cell–cell communication,76,77,78,79,80,81,82 but their roles and underlying mechanisms in the remodeling of subchondral bone during the OA process are not well known. Recently, Liu et al. harvested subchondral bones from OA patients with different severities of joint wear and obtained osteoblasts from them.135 They determined that osteoblast cells from OA subchondral bones could produce exosome-like EVs 30~150 nm in diameter containing exosomal markers (CD9, HSP70, and Flotillin-1).135 Moreover, these exosomes from the osteoblast cells of subchondral bones (SB-OC-EXOs) from OA patients contained high levels of hsamiR-4717-5p, hsa-miR-885-3p, hsa-miR-135a-3p, hsamiR-210-5p, and hsa-miR-1225-5p.135 The physiological and pathological effects of SB-OC-EXOs need further investigation. In addition, exosomes from other cells of subchondral bones are also worthy of attention.
Infrapatellar fat pad-derived exosomes
Infrapatellar fat pad (IPFP), also termed Hoffa’s fat pad, is a knee adipose tissue that plays important roles in knee joint function and pathology.136,137 The size of the IPFP is closely related to knee cartilage volume and structural abnormalities in the clinic.138 In addition, an increasing number of studies have identified IPFP as an emerging source of inflammation that could contribute to OA progression, including pathological severity and knee pain.136,137,139,140,141 Moreover, IPFP-derived MSCs (IPFP-MSCs) presented potent capability for cartilage regeneration in vitro and in vivo, suggesting that these stem cells are promising cell sources for OA treatment.142,143,144,145 Recently, we separated and identified exosomes from IPFP-MSCs (MSC(IPFP)-Exos).146 Our data demonstrated that MSC(IPFP)-Exos can ameliorate gait abnormalities in OA mice and alleviate articular cartilage lesions in vivo.146 Exosomal RNA-seq revealed that miR-100-5p was highly abundant in MSC(IPFP)-Exos.146 MSC(IPFP)-Exos may regulate the biological behaviors of chondrocytes via miR-100-5p-mediated inhibition of mTOR signaling.146 In addition to exosomes from MSCs, the physiological and pathological effects of exosomes from other IPFP-derived cells, including adipocytes, macrophages, lymphocytes, and granulocytes, should also be investigated in the future.
Tendon-derived exosomes
The tendon is an important part of the joint structure that maintains joint stability and regulates the range of motion. Abnormalities of the tendon have been observed during OA progression and shown to be related to OA development.147,148,149 Ibrahim et al. found increased tendon degeneration in patients with shoulder OA.147 The samples from OA-derived tendons presented a degenerative appearance with increased scar tissue and noncollagenous ECM.149 Failed repair of the subscapularis tendon (SSC) significantly increased the risk of developing secondary glenohumeral OA,148 which indicates that enhancement of tendon repair may be a potential strategy for preventing secondary OA after joint injury. Recently, some studies revealed that tendon-derived exosomes could promote tendon repair and regeneration.131,150 Xu et al. reported that tenocytes could secrete exosomes that promote the tenogenic differentiation of MSCs.131 Moreover, the tenogenic differentiation of MSCs induced by tenocyte-derived exosomes could be blocked by the inhibition of TGF-beta signaling.131 In addition, Wang et al. harvested exosomes from conditioned culture medium of tendon stem cells (TSCs) using ultra-high-speed gradient centrifugation and estimated the effect of these exosomes on tendon injury healing.150 Their experimental results showed that TSC-derived exosomes significantly enhanced tendon matrix maintenance in vitro and increased the biomechanical characteristics of ultimate tension and maximal charging in a rat Achilles tendon tendinopathy model.150 More research is needed on role of tendon-derived exosomes in OA cartilage or other tissues.
Ligament-derived exosomes
The ligament is an important intraarticular structure that greatly contributes to joint function. A previous study revealed that the degeneration of the intraarticular ligament was highly associated with cartilage and bone damage during the OA process.151 Destruction of ligament integrity, such as the cruciate ligament, seriously decreased joint stability and promoted the development of knee OA, indicating an important role of the ligament in OA disease.152,153,154 Zhao et al. isolated exosomes from periodontal ligament stem cells using an ultracentrifugation method and identified their exosomal characteristics by TEM, western blot and nanosight tracing analysis.155 Cyclic stretch force promoted the secretion of exosomes from periodontal ligament cells (PDL cells), and these PDL-derived exosomes inhibited the production of IL-1beta in macrophages through the NF-kappaB signaling pathway.156 In addition, Zhao et al. also revealed that human PDL fibroblasts (hPDLFs) could produce exosomes.157 The hPDLF-derived exosomes promoted the inflammatory response and inhibited the osteogenic activity of osteoblasts, which affected bone remodeling in vitro.157 In addition to the PDL, exosomes from joint ligaments, such as the anterior cruciate ligament of the knee joint, should be further studied. Moreover, it is worth exploring the underlying mechanisms of ligament-derived exosomes in joint diseases, including OA.
The therapeutic effects of stem cell-derived exosomes on OA
Stem cells such as bone marrow mesenchymal stem cells (BMSCs) and adipose mesenchymal stem cells (AMSCs) have shown potent cartilage regeneration ability and have been clinically tested for OA treatment.158,159,160 Clinical trials of intra-articular injection of MSCs into the knee with OA have exhibited reliable safety and feasibility,161,162 while this approach could ameliorate the knee society clinical rating system (KSS) and OA outcome score163,164 and partially alleviate knee pain.165,166,167,168 However, the detailed mechanisms for this and other stem cell-based OA treatments have not been well clarified. An increasing number of studies have suggested that the therapeutic effects of stem cells are mainly dependent on the paracrine function of stem cells, including the secretion of EVs.160,169,170 To date, exosomes from different types of stem cells have been revealed to regulate cartilage regeneration and attenuate OA progression in certain models (Fig. 3, Table 2).
Exosomes from bone mesenchymal stem cells
The exosomes derived from BMSCs could affect cell fate, including apoptosis, proliferation, invasion, and migration.171,172 Moreover, BMSC-derived exosomes can regulate a variety of physiological and pathological processes, including the immune response, osteogenesis, fibrosis, and angiogenesis.94,173,174,175 Several studies have reported that BMSC-derived exosomes significantly promote the regeneration and repair of injured tissues, including cartilage and subchondral bone.174,176,177,178,179,180,181 The exosomes as well as MVs/microparticles (MPs) from TGFβ3-pretreated BMSCs significantly increased the expression of anabolic markers and decreased the levels of catabolic marker genes in osteoarthritic chondrocytes.182 In addition, these BMSC-derived exosomes could prevent osteoarthritic chondrocytes from undergoing apoptosis.182 Qi et al. observed that BMSC exosomes could be taken up by chondrocytes to abolish IL-1β-induced cell apoptosis and damage to the mitochondrial membrane potential, in which p38, ERK, and Akt pathways were involved.183 Chen et al. also reported that chondrocytes could take up BMSC exosomes labeled with Dio fluorescent dye.184 These Dio-labeled exosomes were mainly localized in the perinuclear region of chondrocytes and could be fused with the mitochondria.184 Their data also suggested that BMSC-derived exosomes could rescue mitochondrial dysfunction in degenerative chondrocytes. In addition, BMSC-derived exosomes could suppress the activity of osteoclasts in subchondral bone via inhibition of the RANKL-RANK-TRAF6 signaling pathway.185
Moreover, BMSC-derived exosomes could also regulate the biological phenotypes of other OA-related cells, including SFB and macrophages.186 Jin et al. revealed that human BMSC-derived exosomes inhibited the proliferation and enhanced the apoptosis of IL-1β-treated SFB via a miRNA-26a-5p-mediated decrease in PTGS2.186 BMSC-derived exosomes decreased the percentages of F4/80+ macrophages that expressed CD86, MHCII, or CD40 markers. These exosomes also markedly downregulated the level of TNF-α and upregulated that of IL-10, suggesting that BMSC exosomes could inhibit the activation of macrophages in vitro.182
In the collagenase-induced murine model, intra-articular injection of BMSC exosomes reduced articular cartilage damage and subchondral bone degradation.182 Similarly, the damage to cartilage and subchondral bone in the lumbar facet joint (LFJ) OA model can be largely abrogated by BMSC exosomes.185 Moreover, BMSC-derived exosomes could inhibit aberrant nerve invasion and the abnormal formation of H-type vessels in the subchondral bone to relieve pain in LFJ OA mice.185 In an experimental rat OA model, hBMSC-derived exosomes decreased synovial tissue proliferation and inflammatory cell infiltration, downregulating the levels of proinflammatory factors and alleviating pathological changes in the synovium.186 Most current studies in animal OA models mainly focus on the analysis of pathological changes caused by BMSC-derived exosomes, but there is still a lack of assessments of behavioral changes in these models.
Drug intervention or gene modification could regulate the secretion and contents of exosomes, which may influence the effects of exosomes on their targeted cells.43,134,187,188,189 Mao et al. investigated the influence of exosomes from miR-92a-3p-overexpressing BMSCs on chondrogenesis and cartilage degeneration.190 The BMSCs were transfected with miR-92a-3p mimic, and then the exosomes were collected and named MSC-miR-92a-3p-Exos.190 They found that MSC-miR-92a-3p-Exos significantly upregulated the levels of aggrecan, SOX9, COL9A1, COL2A1, and COMP and downregulated the expression of COL10A1, RUNX2 and MMP13, suggesting that BMSC-derived exosomes promote chondrogenesis and prevent cartilage matrix degradation in a miR-92a-3p-dependent manner.190 Moreover, the MSC-miR-92a-3p-Exos could effectively protect the articular cartilage from damage and delay the progression of early OA in a collagenase-induced mouse model.190 In addition, exosomes derived from kartogenin-preconditioned BMSCs were reported to be more effective for cartilage repair and matrix formation in vitro and in vivo than exosomes from BMSCs without kartogenin treatment.191 Therefore, the regulation of exosomal secretion and contents by drug intervention or gene modification could be a potential strategy to enhance the therapeutic effectiveness of BMSC-derived exosomes for OA.
Exosomes from synovial mesenchymal stem cells
Synovial mesenchymal stem cells (SMSCs) have shown preferable chondrogenic differentiation capacity in vitro.192,193,194 Koizumi et al. also reported that SMSCs from OA or RA patients could efficiently enhance cartilage repair using allogenic tissue engineered constructs in vitro and in vivo.195 Intra-articular injection of SMSCs could markedly promote cartilage repair and be used to treat joint-related diseases, including OA, in experimental animal models.196,197,198,199
Recently, several studies revealed that exosomes derived from SMSCs could effectively promote cartilage regeneration and attenuate OA progression.122,195,200 Tao et al. reported that SMSC-derived exosomes could induce the proliferation and emigration of articular chondrocytes in a Wnt5a/Wnt5b/YAP-dependent manner. However, these SMSC exosomes decreased ECM secretion. Exosomes derived from SMSCs transfected with miR-140-5p (SMSC-140-Exos) not only promoted the proliferation and migration of chondrocytes but also prevented ECM damage in vitro.122 Moreover, SMSC-140-Exo treatment significantly lessened joint wear, decreased OARSI scores and delayed OA progression in a rat OA model.122 Similarly, Zhu et al. found that exosomes isolated from human synovial membrane-derived mesenchymal stem cells (SMMSCs) promoted the migration and proliferation of chondrocytes in vitro.200 Moreover, SMMSC exosomes (SMMSC-Exos) elevated ICRS scores, decreased OARSI scores and maintained the collagen II content in a collagenase-induced mouse OA model.200 In addition to cartilage homeostasis, exosomes derived from SMMSCs can also regulate bone remodeling in vitro and in vivo.201 It was reported that SMMSC-derived exosomes decreased glucocorticoid (GC)-induced fatty cell accumulation, bone marrow necrosis, and trabecular bone loss.201 Micro-CT analysis also revealed that SMMSC-Exos significantly improved the microstructures of trabecular bone and mineral density in GC-induced ONFH (femoral head osteonecrosis) in rats.201 In addition, SMMSC-derived exosomes partially reversed GC-induced proliferation arrest and the apoptosis of BMSCs in vitro.201 As bone remodeling, including subchondral bone change and osteophyte formation, is commonly observed during the OA process and is closely related to OA progression, the effects of exosomes derived from SMMSCs or other cells on bone remodeling in OA pathogenesis are worth investigating.
Exosomes from adipose tissue mesenchymal stem cells
Adipose tissue mesenchymal stem cells (AMSCs) were demonstrated to have potent capability for cartilage regeneration and inflammatory modulation and are considered an excellent cell source for OA treatment.202,203,204,205,206,207 However, the mechanisms of AMSC-induced cartilage regeneration are still not clear. Increasing evidence suggests that AMSCs may prevent cartilage erosion and improve joint function primarily through the paracrine secretion of trophic factors to regulate the local microenvironment, making it more favorable for repair and regeneration.205 Tofino-Vian et al. reported that EVs, including MVs and exosomes, mainly mediate the paracrine effects of AMSCs on osteoarthritic osteoblasts.208 The MVs and exosomes from human AMSCs significantly decreased the activity of senescence-associated β-galactosidase and γH2AX foci accumulation, reduced IL-6 and PGE2 levels, enhanced the release of IL-10, and downregulated mitochondrial membrane potential in IL-1β-treated osteoblasts.208 Moreover, AMSC-derived MVs and exosomes could inhibit the production of proinflammatory mediators, such as TNF-α, IL-6, PGE2, and NO, and MMP activity and MMP-13 levels while increasing the levels of the anti-inflammatory cytokine IL-10 and chondrocyte-specific molecule collagen II in OA chondrocytes,209 which suggested potential anti-inflammatory and chondroprotective effects of AMSC-derived MVs and exosomes.209 These findings provide a new perspective for developing therapeutic approaches for OA based on AMSC-derived EVs.
Recently, our group isolated and identified IPFP MSC-derived exosomes.146 We found that MSC(IPFP)-Exos could significantly ameliorate the pathological severity of articular cartilage and partially improve the abnormal gait in DMM-induced OA mice by regulating cartilage homeostasis.146 miR-100-5p was abundant in MSC(IPFP)-Exos and may mediate the inhibition of mTOR and autophagy enhancement by binding to the 3′-untranslated region of mTOR.146 Similarly, Woo et al. reported that EVs (mean diameter of 86.46 nm) from human adipose-derived stem cells (hASCs) enhanced the proliferation and migration of human OA chondrocytes and maintained the chondrocyte matrix.210 Intra-articular injection of hASC-EVs could delay OA progression and protect cartilage from degeneration in both MIA rat and DMM mouse models.210 In addition, Zhao et al. isolated ADSCs from donor adipose tissue after elective liposuction surgery and collected exosomes from these cells (ADSCs-Exos).211 They found that ADSCs-Exos could downregulate the levels of proinflammatory genes and upregulate the expression of anti-inflammatory cytokines in activated SFB, enhancing the proliferation and chondrogenic potential of periosteal cells via upregulation of miR145 and miR221.211 Collectively, these studies further support AMSC-derived exosomes as a potential therapeutic solution for OA in the future.
Exosomes from embryonic mesenchymal stem cells
Embryonic MSCs have been regarded as another potential candidate for cartilage regeneration and OA treatment.212,213,214,215,216 Recently, some studies revealed that exosomes from embryonic MSCs evidently regulated the biological phenotypes of chondrocytes and delayed OA progression in experimental OA models.217,218,219,220,221 In 2016, Zhang et al. successfully isolated and identified exosomes from HuES9 human embryonic stem cells (hESCs).217 After intra-articular injections of hESC-derived exosomes into osteochondral defects in rats, they observed that the damage to cartilage and subchondral bone was largely reversed at 6 weeks and almost completely restored at 12 weeks, indicating that embryonic MSC exosomes (MSC-Exos) could be a cell-free therapeutic alternative for cartilage repair and joint-related disease.217 Similarly, exosomes from deathless E1-MYC 16.3 human embryonic stem cell-derived MSCs enhanced integration and surface regularity with neighboring host cartilage at 12 weeks and 6 weeks in a rat osteochondral defect model.218 Moreover, these exosomes significantly promoted neotissue formation and extracellular matrix deposition, increased M2 macrophage infiltration, and reduced M1 macrophage and proinflammatory cytokine production as early as 2 weeks.218 This study also demonstrated that embryonic MSC-derived exosomes could be endocytosed by chondrocytes to regulate chondrocyte migration, proliferation and matrix synthesis through adenosine-dependent AKT and ERK signaling pathways.218 In the DMM-induced OA model, exosomes from human embryonic stem cell-induced mesenchymal stem cells (ESC-MSCs) significantly prevented cartilage destruction and matrix degradation after 4 weeks of intra-articular injection.219 In addition, exosomes from embryonic MSCs could reduce the inflammatory response, relieve early pain, and promote the repair of cartilage and healing of subchondral bone in an OA model of the temporomandibular joint of immunocompetent rats.220 Embryonic MSC-derived exosomes reversed the IL-1β-mediated inhibition of s-GAG synthesis and reduced the IL-1β-induced production of nitric oxide and MMP13 via adenosine-mediated activation of AKT, ERK, and AMPK.220 Furthermore, TGF-β1 enhanced miR-135b expression in embryonic MSC-derived exosomes, which may promote chondrocyte proliferation by decreasing the expression of Sp1 and accelerating cartilage repair in a rat OA model.221
Other stem cell-derived exosomes in OA
Recently, Zavatti, M. et al. explored the effect of amniotic fluid stem cell (AFSC)-derived exosomes on cartilage repair in an MIA-induced animal OA model.222 The AFSC exosomes enhanced pain tolerance and induced an almost complete restoration of hyaline cartilage with good surface regularity.222 They also observed that AFSC exosomes inhibit M1 polarization, indicating role of AFSC exosomes in regulating inflammation in OA treatment.222 Yan et al. revealed that exosomes from umbilical MSCs (U-MSC-Exos) had potent chondroprotective effects, including stimulating chondrocyte proliferation and migration, increasing matrix synthesis and inhibiting chondrocyte apoptosis.223 Intra-articular injection of U-MSC-Exos could significantly promote the repair of cartilage defects in vivo.223 Moreover, their data also showed that the U-MSC-Exos produced from 3D culture exerted a stronger effect on cartilage repair than those from conventional 2D culture.223 In addition, the exosomes of stem cells from human exfoliated deciduous teeth (SHEDs) have been revealed to inhibit inflammatory reactions and maintain anabolism homeostasis in temporomandibular joint chondrocytes via miR-100-5p-mediated mTOR inhibition.224 The exosomes produced by induced pluripotent stem cell-derived MSCs (iMSC-Exos) were isolated using an ultrafiltration method and identified by western blot assay, tunable resistive pulse-sensing analysis and TEM.200 iMSC-Exos with a diameter of 50–150 nm significantly promoted chondrocyte migration and proliferation in vitro and had a stronger therapeutic effect on OA collagenase-induced mice than SMMSC-Exos, indicating that iMSC-Exos would be more ideal for future use in OA treatment.200
The exosomal contents in OA
The exosomal contents in OA are the basis of exosome physiological function in intercellular communication and signal transduction.28 The contents, including microRNA, long noncoding RNA, DNA, lipid, and protein, could vary with disease progression and greatly contribute to pathological changes in disease.64,225 In addition, the exosomal contents were also closely related to the therapeutic effect of MSC-derived exosomes on the repair and regeneration of injured tissues.226,227 To date, increasing work has revealed the important roles and underlying mechanisms of exosomal contents during the OA process, including miRNAs, lncRNAs, and proteins. However, other types of RNA and the lipid composition of exosomes in OA pathology have not been reported and need to be further investigated.
Exosomal miRNA in OA
The miRNA content of SF exosomes differed between OA- and non-OA-derived samples.99 There is significant gender-specific differential expression of exosome miRNAs in SF from OA patients.99 In the male group, 69 miRNAs, such as miR-6878-3p, were downregulated, while 45 miRNAs, including miR-210-5p, were upregulated in exosomes isolated from OA-derived SF. In the female group, 91 miRNAs, including miR26a-5p, miR-146a-5p, and miR-6821-5p, were decreased, while 53 miRNAs, including miR-16-2-3p, were increased.99 GO and KEGG analyses revealed that specific exosomal miRNAs from female OA SF are closely associated with the estrogen response and TLR (toll-like receptor) signaling pathways.99 Mao et al. detected the expression profiles of miRNAs in exosomes derived from OA and normal chondrocytes by miRNA microarray.109 They found that the expression of 22 miRNAs was markedly increased, while the expression of 29 other miRNAs was decreased in OA chondrocyte-secreted exosomes in comparison with that in normal chondrocytes.109 Compared with normal chondrocytes, exosomal miR-92a-3p190 and miR-95-5p109 secreted from OA chondrocytes were significantly downregulated. miR-146a-5p was found to be enriched in the EVs of adipose-derived MSCs (ASCs) cultured under inflammatory OA SF conditions and may contribute to OA progression.228 In addition, IL-1β treatment could change the miRNA profile of exosomes from human SFB, including the upregulation of 11 miRNAs and downregulation of 39 miRNAs.121 Using high-throughput sequencing, 124 differentially expressed miRNAs were identified by screening in OA patient-derived subchondral osteoblasts. Among these miRNAs, hsa-miR-4717-5p had the maximum fold change and targeted a multifunctional regulator of G-protein signaling RGS2.135 These studies indicate that exosomal miRNAs from different joint tissues vary with OA disease and are potential candidates for diagnostic OA markers and therapeutic targets.
Exosomal miRNAs play an important role in the therapeutic effects of exosomes on OA (Table 3). Jin et al. reported that miRNAs could be successfully transferred from MSCs to OA-related cells, such as SFB, through exosomes.186 The overexpression of miR-26a-5p could regulate hBMSC-exosome function to promote the survival of SFB via downregulation of PTGS2 in vitro and reduce synovitis in a rat OA model.186 TGF-β1 could increase the level of miR-135b in MSC-Exos; these exosomes thus showed enhanced stimulation of chondrocyte proliferation in vitro by negatively regulating Sp1 expression and may subsequently promote cartilage repair in vivo.221 The exosomes from anti-miR-92a-3p-transfected MSCs inhibited chondrogenic differentiation and the synthesis of cartilage matrix by upregulating the level of WNT5A, while the exosomes from miR-92a-3p-transfected MSCs significantly promoted cell proliferation and increased matrix synthesis in primary OA human chondrocytes.190 The exosomes produced by IPFP-MSCs contained abundant miR-100-5p that can bind to the 3′-untranslated region (3′UTR) of mTOR to decrease its expression.146 The decreased mTOR may initiate autophagy and restore cartilage homeostasis in vitro and vivo.146,229,230,231 Similarly, miR-100-5p was also enriched in the exosomes of stem cells isolated from human exfoliated deciduous teeth and participated in the suppression of inflammation in TMJ OA chondrocytes via directly targeting mTOR signaling.224 The exosomes from miR-140-5p-overexpressing SMSCs (SMSC-140-Exos) could activate the YAP signal and promote the proliferation and migration of chondrocytes without decreasing ECM secretion. These SMSC-140-Exo-mediated effects were mainly dependent on the miR-140-5p/RalA/SOX9 signaling pathway.122 The exosomes from normal chondrocytes transfected with miR-95-3p mimicked binding to the 3ʹ-UTRs of HDAC2/8, and maintained cartilage development and homeostasis via the miR-95-3p-mediated transcriptional inhibition of HDAC2/8.109 In addition to its protective roles, the miRNA also contributes to the effects of exosomes on joint inflammation and OA progression. The exosomes from osteoarthritic chondrocytes (OC-Exos) could stimulate IL-1beta processing and production in macrophages via miR-449a-5p-mediated autophagy inhibition.107 miR-449a-5p can bind to the 3′-UTR of the ATG4B gene to inhibit its expression, which leads to autophagy inhibition and inflammasome activation of macrophages in vitro and may ultimately promote synovitis and cartilage erosion in DMM-induced mice.107 Antagonizing miR-449a-5p reversed OC-Exo-mediated proinflammatory effects and cartilage destruction, which provided a new perspective for OA treatment based on miR-449a-5p of OC-Exos.107
The exosomal lncRNAs in OA
Long noncoding RNAs (lncRNAs) are important exosomal contents that participate extensively in the regulation of a wide range of pathological and physiological processes.232 Exosomal lncRNAs, such as lncRNA CRNDE-h and lncRNA-p21, are considered possible biomarkers for carcinoma diagnosis and prognosis.233,234 Recently, Zhao et al. harvested SF samples from prearthritic patients, early-stage OA patients and late-stage OA patients.101 The exosomes from these samples were extracted by ultracentrifugation and subjected to RNA detection.101 They found that the exosomal lncRNA PCGEM1 was gradually elevated with OA progression and exhibited a positive relationship with the WOMAC Index (the Western Ontario and McMaster Universities Osteoarthritis Index), suggesting that exosomal lncRNA PCGEM1 could be a potent indicator for OA diagnosis and recognizing the difference between early OA and late OA in clinical practice.101 In addition, exosomes can modulate multiple cell phenotypes, including tissue repair and regeneration, via exosomal lncRNAs.235,236,237,238 Liu et al. reported that MSC-Exos upregulated Col2a1 and aggrecan levels, downregulated MMP13 and Rux2 expression, and promoted the survival of IL-1β-treated chondrocytes mainly through exosomal lncRNA-KLF3-AS1.239 The lncRNA KLF3-AS1 enhanced the expression of GIT1 by sponging miR-206 in vitro.240 The overexpression of miR-206 or knockdown of GIT1 partially attenuated MSC(KLF3-AS1)-Exo-mediated effects on chondrocyte repair.240 These studies suggested that exosomal lncRNA-KLF3-AS1 plays an important role in the effect of MSC-Exos on osteoarthritic chondrocytes mainly through targeting the miR-206/GIT1 axis.240 Moreover, exosomal lncRNA-KLF3-AS1 significantly contributed to MSC-exo-mediated cartilage repair in a collagenase II-induced OA model.239
The exosomal proteins in OA
Several types of specific cellular proteins can be selectively sorted into MVBs and secreted as exosome loading cargo.241,242,243 Exosomal proteins have been demonstrated to contribute to cell–cell communication and signal transduction, which are highly involved in various diseases.244,245,246 The synovial exosomes from RA patients contained potential autoantigenic content, including citrullinated proteins, which may participate in the autoimmune reaction.97 Tsuno et al. extracted serum exosomal proteins from the healthy group, OA group and RA group and identified the exosomal proteins via 2D-DIGE and mass spectrometry methods.247 They found that the exosomal protein profiles from the serum of the three groups were significantly different. There were 21 spots in the exosomal protein profiles with different intensities between the OA group and the healthy group, such as cathepsin F and Igalpha-2 chain C region, indicating the potential roles of these proteins in OA.247 Moreover, exosomal proteins have been revealed to participate in the exosome-mediated regulation of the biological response in chondrocytes. CD73/ecto-5′-nucleotidase was expressed in MSC-Exos. The CD73 inhibitor AMPCP or the nonselective adenosine receptor antagonist theophylline could reduce MSC exosome-induced AKT and ERK phosphorylation in chondrocytes, indicating that exosomal CD73/ecto-5′-nucleotidase played an important role in the biological effect of MSC-Exos on cartilage.218
Perspective
Exosomes may become potent candidate risk factors and early diagnostic markers for clinical OA patients in the future. Subgroups of exosomes distinguished by the levels or activity of exosome-specific proteins were highly associated with cancer onset and progression and can be used to detect early cancer.63,248 In addition, miRNAs and lncRNAs in exosomes have also been considered as potential diagnostic markers for several kinds of diseases, including RA and OA.99,101,234,249,250,251 More studies are needed to identify the different subgroups of exosomes from OA peripheral blood or SF samples and analyze their relationship with OA pathological changes and clinical classification. In addition, we can attempt to identify the original secreted cells of exosomes based on exosome-specific markers. Moreover, urine can be harvested through noninvasive approaches, which may be more acceptable for patients in clinical practice. Previous studies have revealed that the components of urine were changed with OA progression and could predict cartilage degradation as a biochemical marker, indicating that urine could be used to evaluate joint-related diseases.252,253,254 In addition, the contents from urine exosomes are highly relevant to several types of disorders, including cancer, and could be used as potential diagnostic markers for these diseases.255,256,257 Therefore, exosomes from body fluids other than blood, such as urine, should be given additional attention to explore their availability as diagnostic markers for OA patients.
The endogenous exosomes in our body have many physiological and pathological functions and have been considered potent therapeutic targets in a variety of diseases.258,259,260 The roles and underlying mechanisms of exosomes from different joint tissues in OA pathogenesis are quite variable and could be affected by many factors, including sex, obesity, aging, basic diseases, therapeutic intervention, joint motion, and microenvironment. For example, exosomes from chondrocytes cultured in a normal environment can increase M2 macrophage infiltration and delay OA progression,106 while exosomes from IL-1β-treated chondrocytes promote inflammasome activation in macrophages and aggravate OA pathological severity.107 Intensive studies of endogenous exosomes originating from joint cells during the OA process would be beneficial for developing novel targeted therapeutic strategies. More investigations should aim to reduce the release of pro-OA (promoting OA onset and progression) exosomes and the pro-OA contents of these exosomes or inhibit exosome-mediated signal transduction in targeted cells. Conversely, some measures could be taken to increase the production of endogenous anti-OA (inhibiting OA onset and progression) exosomes, such as IPFP MSC-Exos and synovial MSC-Exos,146,197 and to enhance their anti-OA capacity by acting on the target cells.
To date, several types of exosomes derived from MSCs of different tissues have exhibited protective effects on cartilage in vitro and in vivo. However, the separation methods for these MSC-derived exosomes are quite different (Table 3) and have some disadvantages, which may influence clinical standardization and potential application. Ultracentrifugation, the most classic separation method, usually requires extended time to harvest exosomes in vitro, which may increase the risk of infection. PEG-mediated isolation of exosomes may influence the biological activity of exosomes for subsequent clinical application. Chen et al. compared the efficiency of three separation techniques (ultracentrifugation, filtration combined with size exclusion chromatography and 8% polyethylene glycol) for extracting synovial tissue-derived exosomes (Syn-Exos).261 They found that the efficiency of exosome isolation differed among these three isolation methods and recommended ultracentrifugation and filtration combined with size exclusion chromatography for the extraction of Syn-Exos.261 Therefore, additional measures are needed to optimize and standardize the existing separation methods so that the clinical use of MSC-Exos is safe and convenient.
Regulation of the secretion and contents of exosomes by drug intervention or gene/material modification contributes greatly to the therapeutic action of MSC-Exos in diverse diseases,262,263,264,265 so it is advisable to develop engineering technology for MSC-Exos for OA treatment. Recently, MSC-Exos combined with a hydrogel sponge were used to accelerate wound healing and regeneration in animal injury models, including a cartilage damage model,266,267,268,269 suggesting a potential application of this technology in OA treatment. The hydrogel sponge could make the MSC-Exos more stable in vivo and might better control the release of MSC-Exos according to changes in the joint cavity microenvironment, such as increased proinflammatory factors and matrix metalloproteinase activity or excessive mechanical load. Apart from MSCs, exosomes from other cells or tissues may also have a potential therapeutic effect on OA. A recent study revealed that exosomes derived from platelet-rich plasma (PRP-Exos) can significantly protect cartilage from damage by activating the Wnt/β-catenin signaling pathway, whose therapeutic effect was even better than that of activated PRP.270 Additional research is needed to improve the therapeutic effects of exosomes derived from different sources.
Nevertheless, there are also some challenges and problems in this field. There is still a lack of direct evidence that endogenous exosomes could transfer from one cell to another cell in the joint in vivo, which restricts further studies, such as the identification of the major target cells of different exosomes. Moreover, the mechanisms of exosome generation and release in the joint are still unclear, limiting exosome-based targeted intervention. In addition, there are some difficulties in achieving the protective effects of MSC-Exos on chondrocytes during the early stage of OA. As cartilage is relatively complete at the early OA stage, it may be difficult for exosomes to permeate through the cartilage matrix to enter chondrocytes, especially the chondrocytes in the deep layer. Therefore, the key points of engineering MSC-Exos for use in the early OA stage may focus on superficial chondrocytes, synovial cells, and other joint cells that are easily accessible by exosomes, or on cartilage matrix maintenance.
Conclusions
As an important intercellular communication mediator, exosomes greatly contribute to OA onset and progression and have shown strong potential for OA treatment. Exploring the detailed mechanisms of exosomes in OA pathological changes will help us to screen and identify potential therapeutic targets. Moreover, we need to optimize MSC-Exos to improve their therapeutic effects on OA. Studies on the roles, underlying mechanisms, and diagnostic/therapeutic application of exosomes in OA are only beginning, and there are still many problems to be solved in this field. With advances in technology, we speculate that exosome-based treatment will be applied to OA patients in the future.
References
GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet392, 1923–1994 (2018).
Hawker, G. A., White, D. & Skou, S. T. Non-pharmacological management of osteoarthritis. Osteoarthr. Cartil.25, S4 (2017).
Bannuru, R. R. et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil.27, 1578–1589 (2019).
Kloppenburg, M. & Berenbaum, F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr. Cartil.28, 242–248 (2020).
Dieppe, P., Lim, K. & Lohmander, S. Who should have knee joint replacement surgery for osteoarthritis? Int J. Rheum. Dis.14, 175–180 (2011).
Loeser, R. F., Goldring, S. R., Scanzello, C. R. & Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum.64, 1697–1707 (2012).
Chen, D. et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res.5, 1–13 (2017).
Vina, E. R. & Kwoh, C. K. Epidemiology of osteoarthritis: literature update. Curr. Opin. Rheumatol.30, 160–167 (2018).
Burr, D. B. The importance of subchondral bone in osteoarthrosis. Curr. Opin. Rheumatol.10, 256–262 (1998).
de Lange-Brokaar, B. J. et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthr. Cartil.20, 1484–1499 (2012).
Glyn-Jones, S. et al. Osteoarthritis. Lancet386, 376–387 (2015).
Lin, C. et al. Activation of mTORC1 in subchondral bone preosteoblasts promotes osteoarthritis by stimulating bone sclerosis and secretion of CXCL12. Bone Res.7, 5 (2019).
Zhen, G. et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med.19, 704–712 (2013).
Sharma, A. R., Jagga, S., Lee, S. S. & Nam, J. S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int. J. Mol. Sci.14, 19805–19830 (2013).
Mathiessen, A. & Conaghan, P. G. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res. Ther.19, 18 (2017).
Kuang, L. et al. FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Ann. Rheum. Dis.79, 112–122 (2020).
Clockaerts, S. et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthr. Cartil.18, 876–882 (2010).
Maas, S. L. N., Breakefield, X. O. & Weaver, A. M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol.27, 172–188. (2017).
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol.Chapter 3:Unit 3, 22 (2006).
Trams, E. G., Lauter, C. J., Salem, N. Jr. & Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta645, 63–70 (1981).
Harding, C., Heuser, J. & Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol.97, 329–339 (1983).
Pan, B. T., Teng, K., Wu, C., Adam, M. & Johnstone, R. M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol.101, 942–948 (1985).
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L. & Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem.262, 9412–9420 (1987).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med.183, 1161–1172 (1996).
Wolfers, J. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med.7, 297–303 (2001).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med.4, 594–600 (1998).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol.9, 654–659 (2007).
Mathieu, M., Martinjaular, L., Lavieu, G. & Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol.21, 9–17 (2019).
Pluchino, S. & Smith, J. A. Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell177, 225–227 (2019).
Théry, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol.2, 569–579 (2002).
Batista, B. S., Eng, W. S., Pilobello, K. T., Hendricks-Muñoz, K. D. & Mahal, L. K. Identification of a conserved glycan signature for microvesicles. J. Proteome Res.10, 4624–4633 (2011).
Heijnen, H. F. G., Schiel, A. E., Fijnheer, R., Geuze, H. J. & Sixma, J. J. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood94, 3791–3799 (1999).
Hanson, P. I., Shim, S. & Merrill, S. A. Cell biology of the ESCRT machinery. Curr. Opin. Cell Biol. 21, 568–574 (2009).
Hurley, J. H. & Odorizzi, G. Get on the exosome bus with ALIX. Nat. Cell Biol.14, 654–655 (2012).
Mayers, J. R. & Audhya, A. Vesicle formation within endosomes: an ESCRT marks the spot. Commun. Integr. Biol.5, 50–56 (2012).
Nabhan, J. F., Hu, R., Oh, R. S. & Cohen, S. N. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc. Natl. Acad. Sci. USA109, 4146–4151 (2012).
Henne, W. M., Stenmark, H. & Emr, S. D. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb. Perspect. Biol.5, a016766 (2013).
Raiborg, C. & Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature458, 445–452 (2009).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science367, eaau6977 (2020).
Trajkovic, K. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science319, 1244–1247 (2008).
Yang, D. et al. Progress, opportunity, and perspective on exosome isolation—efforts for efficient exosome-based theranostics. Theranostics10, 3684–3707 (2020).
Li, P., Kaslan, M., Lee, S. H., Yao, J. & Gao, Z. Progress in exosome isolation techniques. Theranostics7, 789–804 (2017).
Gurunathan, S., Kang, M. H., Jeyaraj, M., Qasim, M. & Kim, J. H. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells8, 307 (2019).
Zarovni, N. et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods87, 46–58 (2015).
Jeppesen, D. K. et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles3, 25011 (2014).
Cheruvanky, A. et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol.292, F1657–F1661 (2007).
Heinemann, M. L. et al. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A.1371, 125–135 (2014).
Batrakova, E. V. & Kim, M. S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control Release219, 396–405 (2015).
Yamamoto, K. R., Alberts, B., Benzinger, R., Lawhorne, L. W. & Treiber, G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology40, 734–744 (1970).
Lewis, G. D. & Metcalf, T. G. Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl. Environ. Microbiol.54, 1983–1988 (1988).
Adams, A. Concentration of epstein-barr virus from cell culture fluids with polyethylene glycol. J. Gen. Virol.20, 391–394 (1973).
Wang, Z. et al. Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip.13, 2879–2882 (2013).
Lee, K., Shao, H., Weissleder, R. & Lee, H. Acoustic purification of extracellular microvesicles. ACS Nano.9, 2321–2327 (2015).
Davies, R. T. et al. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip.12, 5202–5210 (2012).
Liga, A., Vliegenthart, A. D. B., Oosthuyzen, W., Dear, J. W. & Kersaudy-Kerhoas, M. Exosome isolation: a microfluidic road-map. Lab Chip.15, 2388–2394 (2015).
Wunsch, B. H. et al. Nanoscale lateral displacement arrays for the separation of exosomes and colloids down to 20 nm. Nat. Nanotechnol.11, 936–940 (2016).
Lässer C., Eldh M., Lötvall J. Isolation and characterization of RNA-containing exosomes. J. Vis. Exp. 59, e3037 (2012).
Wu, Y., Deng, W. & Klinke, D. J. 2nd. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst140, 6631–6642 (2015).
Kalluri, R. & LeBleu, V. S. The biology, function, and biomedical applications of exosomes. Science367, eaau6977 (2020).
Thery, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles7, 1535750 (2018).
Escola, J. M. et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem.273, 20121–20127 (1998).
Thery, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol.2, 569–579 (2002).
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature523, 177–182 (2015).
Ruivo, C. F., Adem, B., Silva, M. L. & Melo, S. A. The biology of cancer exosomes: insights and new perspectives. Cancer Res.77, 6480–6488 (2017).
Danzer, K. M. et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener.7, 42 (2012).
Sardar Sinha, M. et al. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol.136, 41–56 (2018).
Ding, X. et al. Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure. Oncotarget6, 24178–24191 (2015).
Yang, C. & Robbins, P. D. Immunosuppressive exosomes: a new approach for treating arthritis. Int. J. Rheumatol.2012, 573528 (2012).
Kim, S. H. et al. Exosomes Derived from IL-10-Treated Dendritic Cells Can Suppress Inflammation and Collagen-Induced. Arthritis174, 6440–6448 (2005).
Michael, A. et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis.16, 34–38 (2010).
Lai, R. C., Chen, T. S. & Lim, S. K. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen. Med.6, 481–492 (2011).
Bellin, G. et al. Exosome in cardiovascular diseases: a complex world full of hope. Cells8, 166 (2019).
Gao, M. et al. Exosomes-the enigmatic regulators of bone homeostasis. Bone Res.6, 36 (2018).
Rodan, G. A. Bone homeostasis. Proc. Natl Acad. Sci. USA.95, 13361–13362 (1998).
Ge, M., Ke, R., Cai, T., Yang, J. & Mu, X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem. Biophys. Res. Commun.467, 27–32 (2015).
Cui, Y., Luan, J., Li, H., Zhou, X. & Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett.590, 185–192 (2016).
Li, D. et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun.7, 10872 (2016).
Sun, W. et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov.2, 16015 (2016).
Yang, J.-X., Xie, P., Li, Y.-S., Wen, T. & Yang, X.-C. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell. Signal.70, 109504 (2019).
Qin, Y. et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem.292, 11021–11033 (2017).
Kuang, M. J. et al. Exosomes derived from Wharton’s jelly of human umbilical cord mesenchymal stem cells reduce osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head in rats via the miR-21-PTEN-AKT signalling pathway. Int. J. Biol. Sci.15, 1861–1871 (2019).
Ren, L. et al. Adipose mesenchymal stem cell-derived exosomes ameliorate hypoxia/serum deprivation-induced osteocyte apoptosis and osteocyte-mediated osteoclastogenesis in vitro. Biochem. Biophys. Res. Commun.508, 138–144. (2019).
Qi, X. et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci.12, 836–849 (2016).
Zhang, Y. et al. Transfer RNA-derived fragments as potential exosome tRNA-derived fragment biomarkers for osteoporosis. Int. J. Rheum. Dis.21, 1659–1669 (2018).
Jiang, L. B., Tian, L. & Zhang, C. G. Bone marrow stem cells-derived exosomes extracted from osteoporosis patients inhibit osteogenesis via microRNA-21/SMAD7. Eur. Rev. Med. Pharmacol. Sci.22, 6221–6229 (2018).
Zhao, P., Xiao, L., Peng, J., Qian, Y. Q. & Huang, C. C. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci.22, 3962–3970 (2018).
Yang, X., Yang, J., Lei, P. & Wen, T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging11, 8777–8791 (2019).
Yang, B. C. et al. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun.524, 883–889 (2020).
Liu, X. et al. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci.13, 232–244 (2017).
Li, Y. et al. Mesenchymal stem cells-derived exosomes: a possible therapeutic strategy for osteoporosis. Curr. Stem Cell Res. Ther.13, 362–368 (2018).
Hao, Z. C. et al. Stem cell-derived exosomes: a promising strategy for fracture healing. Cell Prolif.50, e12359 (2017).
Liu, W. et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomaterialia.103, 196–212 (2020).
Furuta, T. et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med.5, 1620–1630 (2016).
Zhang, L. et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther.11, 38 (2020).
Zhang, Y. et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif.52, e12570 (2019).
Domenis, R. et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflamm.2017, 4814987 (2017).
Skriner, K., Adolph, K., Jungblut, P. R. & Burmester, G. R. Association of citrullinated proteins with synovial exosomes. Arthritis Rheumatism.54, 3809–3814 (2006).
Gao, K. et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Mod. Rheumatol.2019, 1–7 (2019).
Kolhe, R. et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep.7, 2029 (2017).
Headland, S. E. et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med.7, 315ra190 (2015).
Zhao, Y. & Xu, J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop.42, 2865–2872 (2018).
Anderson, H. C. Matrix vesicles and calcification. Curr. Rheumatol. Rep.5, 222–226 (2003).
Jubeck, B. et al. Promotion of articular cartilage matrix vesicle mineralization by type I collagen. Arthritis Rheumatism.58, 2809–2817 (2008).
Shapiro, I. M., Landis, W. J. & Risbud, M. V. Matrix vesicles: are they anchored exosomes? Bone79, 29–36 (2015).
Mitton, E., Gohr, C. M., McNally, M. T. & Rosenthal, A. K. Articular cartilage vesicles contain RNA. Biochem. Biophys. Res. Commun.388, 533–538 (2009).
Zheng, L. et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomed. (Lond.).14, 3193–3212 (2019).
Ni, Z. et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis.10, 522 (2019).
Chen, Y., Xue, K., Zhang, X., Zheng, Z. & Liu, K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res. Ther.9, 318 (2018).
Mao, G. et al. Exosomal miR-95-5p regulates chondrogenesis and cartilage degradation via histone deacetylase 2/8. J. Cell. Mol. Med.22, 5354–5366. (2018).
Song, J., Kang, Y., Chun, C. H. & Jin, E. J. Selective loading of exosomal HULC and miR-372 is responsible for chondrocyte death during OA pathogenesis. Anim. Cells Syst.21, 397–403 (2017).
Li, Z. et al. Chondrocytes-derived exosomal miR-8485 regulated the Wnt/beta-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem. Biophys. Res. Commun.523, 506–513 (2020).
Baker, K. et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann. Rheum. Dis.69, 1779–1783 (2010).
Benito, M. J., Veale, D. J., FitzGerald, O., van den Berg, W. B. & Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis.64, 1263–1267 (2005).
Hill, C. L. et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis.66, 1599–1603 (2007).
de Lange-Brokaar, B. J. et al. Association of pain in knee osteoarthritis with distinct patterns of synovitis. Arthritis Rheumatol.67, 733–740 (2015).
Robinson, W. H. et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol.12, 580–592 (2016).
Krenn, V. et al. Grading of chronic synovitis–a histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract.198, 317–325 (2002).
Wojdasiewicz, P., Poniatowski, L. A. & Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.2014, 561459 (2014).
Bondeson, J. et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum.62, 647–657 (2010).
Pérez-Garcia, S. et al. Wnt and RUNX2 mediate cartilage breakdown by osteoarthritis synovial fibroblast-derived ADAMTS-7 and -12. J. Cell. Mol. Med.23, 3974–3983 (2019).
Kato, T. et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res. Ther.16, R163 (2014).
Tao, S. C. et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics7, 180–195 (2017).
Tsuno, H. et al. Effects of methotrexate and salazosulfapyridine on protein profiles of exosomes derived from a human synovial sarcoma cell line of SW982. Proteomics Clin. Appl.10, 164–171 (2016).
Castañeda, S., Roman-Blas, J. A., Largo, R. & Herrero-Beaumont, G. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol.83, 315–323 (2012).
Stewart, H. L. & Kawcak, C. E. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front. Vet. Sci.5, 178 (2018).
Maas, O., Joseph, G. B., Sommer, G., Wild, D. & Kretzschmar, M. Association between cartilage degeneration and subchondral bone remodeling in patients with knee osteoarthritis comparing MRI and (99m)Tc-DPD-SPECT/CT. Osteoarthr. Cartil.23, 1713–1720 (2015).
Barr, A. J. et al. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther.17, 228 (2015).
Cui, Z. et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-beta activity and H-type vessel formation in subchondral bone. Ann. Rheum. Dis.75, 1714–1721 (2016).
Zhu, S. et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J. Clin. Invest.129, 1076–1093 (2019).
Chen, Y. et al. Attenuation of subchondral bone abnormal changes in osteoarthritis by inhibition of SDF-1 signaling. Osteoarthr. Cartil.25, 986–994 (2017).
Xu, T. et al. Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-beta. Cytotechnology71, 57–65 (2019).
Zhu, Q. J., Zhu, M., Xu, X. X., Meng, X. M. & Wu, Y. G. Exosomes from high glucose-treated macrophages activate glomerular mesangial cells via TGF-beta1/Smad3 pathway in vivo and in vitro. FASEB J.33, 9279–9290 (2019).
Yao, Y., Chen, R., Wang, G., Zhang, Y. & Liu, F. Exosomes derived from mesenchymal stem cells reverse EMT via TGF-beta1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res. Ther.10, 225 (2019).
Cai, Z. et al. Immunosuppressive exosomes from TGF-beta1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res.22, 607–610 (2012).
Liu, B. et al. [Differential expression of exosomal miRNAs in osteoblasts in osteoarthritis]. Zhong Nan Da Xue Xue Bao Yi Xue Ban43, 1294–1300 (2018).
Gandhi, R. et al. Microarray analysis of the infrapatellar fat pad in knee osteoarthritis: relationship with joint inflammation. J. Rheumatol.38, 1966–1972 (2011).
Ioan-Facsinay, A. & Kloppenburg, M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res. Ther.15, 225 (2013).
Cai, J. et al. Association between infrapatellar fat pad volume and knee structural changes in patients with knee osteoarthritis. J. Rheumatol.42, 1878–1884 (2015).
Ballegaard, C. et al. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: a cross-sectional study. Osteoarthr. Cartil.22, 933–940 (2014).
Belluzzi, E. et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed. Res. Int.2019, 6390182 (2019).
Distel, E. et al. The infrapatellar fat pad in knee osteoarthritis: an important source of interleukin-6 and its soluble receptor. Arthritis Rheum.60, 3374–3377 (2009).
Koh, Y. G. & Choi, Y. J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee19, 902–907 (2012).
Toghraie, F. S. et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee18, 71–75 (2011).
Buckley, C. T. et al. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J. Biomech.43, 920–926 (2010).
Buckley, C. T., Vinardell, T. & Kelly, D. J. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthr. Cartil.18, 1345–1354 (2010).
Wu, J. et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials206, 87–100 (2019).
Ibrahim, M., Kartus, J. T., Steigen, S. E., Olsen, R. & Meknas, K. More tendon degeneration in patients with shoulder osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc.27, 267–275 (2019).
Plachel, F. et al. Repair failure increases the risk of developing secondary glenohumeral osteoarthritis: A long-term follow-up after open repair of large subscapularis tendon tears. Orthop. Traumatol. Surg. Res.105, 1529–1533 (2019).
Meknas, K. et al. Could tendinosis be involved in osteoarthritis? Scand. J. Med. Sci. Sports22, 627–634 (2012).
Wang, Y. et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J. Cell. Mol. Med.23, 5475–5485 (2019).
Schulze-Tanzil G. Intraarticular ligament degeneration is interrelated with cartilage and bone destruction in osteoarthritis. Cells.8, 990 (2019).
Hill, C. L. et al. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum.52, 794–799 (2005).
Johnson, V. L. et al. Loss of anterior cruciate ligament integrity and the development of radiographic knee osteoarthritis: a sub-study of the osteoarthritis initiative. Osteoarthr. Cartil.23, 882–887 (2015).
Davis, J. E. et al. Accelerated knee osteoarthritis is associated with pre-radiographic degeneration of the extensor mechanism and cruciate ligaments: data from the Osteoarthritis Initiative. BMC Musculoskelet. Disord.20, 308 (2019).
Zhao, L. R., Mao, J. Q., Zhao, B. J. & Chen, J. [Isolation and biological characteristics of exosomes derived from periodontal ligament stem cells]. Shanghai Kou Qiang Yi Xue28, 343–348 (2019).
Wang, Z. et al. Cyclic stretch force induces periodontal ligament cells to secrete exosomes that suppress IL-1beta production through the inhibition of the NF-kappaB signaling pathway in macrophages. Front. Immunol.10, 1310 (2019).
Zhao, M. et al. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch. Oral. Biol.105, 27–34 (2019).
Kong, L., Zheng, L. Z., Qin, L. & Ho, K. K. W. Role of mesenchymal stem cells in osteoarthritis treatment. J. Orthop. Translation.9, 89–103 (2017).
Lee, W. Y. & Wang, B. Cartilage repair by mesenchymal stem cells: Clinical trial update and perspectives. J. Orthop. Translation.9, 76–88 (2017).
De Bari, C. & Roelofs, A. J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol.40, 74–80 (2018).
Di Matteo, B. et al. Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cells Int.2019, 1735242 (2019).
Wang, W. & Cao, W. Treatment of osteoarthritis with mesenchymal stem cells. Sci. China Life Sci.57, 586–595 (2014).
Jo, C. H. et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am. J. Sports Med.45, 2774–2783 (2017).
Kim S. H., et al. Intra-articular injection of culture-expanded mesenchymal stem cells without adjuvant surgery in knee osteoarthritis: a systematic review and meta-analysis. Am. J. Sports Med. 363546519892278 (2019).
Garay-Mendoza, D. et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int. J. Rheum. Dis.21, 140–147 (2018).
Jo, C. H. et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells32, 1254–1266 (2014).
Yokota, N. et al. Comparative clinical outcomes after intra-articular injection with adipose-derived cultured stem cells or noncultured stromal vascular fraction for the treatment of knee osteoarthritis. Am. J. Sports Med.47, 2577–2583 (2019).
Shin, Y. S., Yoon, J. R., Kim, H. S. & Lee, S. H. Intra-articular injection of bone marrow-derived mesenchymal stem cells leading to better clinical outcomes without difference in MRI outcomes from baseline in patients with knee osteoarthritis. Knee Surg. Relat. Res.30, 206–214 (2018).
Meirelles Lda, S., Fontes, A. M., Covas, D. T. & Caplan, A. I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev.20, 419–427 (2009).
Phinney, D. G. & Pittenger, M. F. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells35, 851–858 (2017).
Xu, Y. et al. microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J. Cell. Physiol.234, 21380–21394 (2019).
Xie, C., Du, L. Y., Guo, F., Li, X. & Cheng, B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell. Biochem.458, 11–26 (2019).
He J. G., Xie Q. L., Li B. B., Zhou L., Yan D. Exosomes derived from IDO1-overexpressing rat bone marrow mesenchymal stem cells promote immunotolerance of cardiac allografts. Cell Transplant.27, 1657–1683 (2018).
Zhao, C. et al. Exosomes derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord injury. Drug Des. Dev. Ther.13, 3693–3704 (2019).
Rong, X. et al. Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/beta-catenin pathway. Stem Cell Res. Ther.10, 98 (2019).
Ding, J., Wang, X., Chen, B., Zhang, J. & Xu, J. Exosomes derived from human bone marrow mesenchymal stem cells stimulated by deferoxamine accelerate cutaneous wound healing by promoting angiogenesis. BioMed. Res. Int.2019, 9742765 (2019).
Xiong L., et al. Exosomes from bone marrow mesenchymal stem cells can alleviate early brain injury after subarachnoid hemorrhage through miRNA129-5p-HMGB1 pathway. Stem Cells Dev.29, 212–221 (2020).
Zhu, G. et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell. Physiol.234, 23736–23749 (2019).
Zhao, L. et al. Exosomes derived from bone marrow mesenchymal stem cells overexpressing microRNA-25 protect spinal cords against transient ischemia. J. Thorac. Cardiovasc. Surg.157, 508–517 (2019).
Asghar, S., Litherland, G. J., Lockhart, J. C., Goodyear, C. S. & Crilly, A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology59, 57–68 (2020).
Mianehsaz, E. et al. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res. Ther.10, 340 (2019).
Cosenza, S., Ruiz, M., Toupet, K., Jorgensen, C. & Noel, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep.7, 16214 (2017).
Qi, H. et al. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. Vitr. Cell. Develop. Biol. Anim.55, 203–210 (2019).
Chen, P. et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics9, 2439–2459 (2019).
Li, J. et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. J. Orthop. Res.38, 670–679 (2019).
Jin, Z., Ren, J. & Qi, S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol.78, 105946 (2020).
Ma, J. et al. Exosomes derived from akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl. Med.6, 51–59 (2017).
Shelton, M. N., Huang, M. B., Ali, S. A., Powell, M. D. & Bond, V. C. Secretion modification region-derived peptide disrupts HIV-1 Nef’s interaction with mortalin and blocks virus and Nef exosome release. J. Virol.86, 406–419 (2012).
Liu, C. & Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics9, 1015–1028 (2019).
Mao, G. et al. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther.9, 247 (2018).
Liu, C. et al. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomed. (Lond.).15, 273–288 (2020).
Kurth, T. et al. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthr. Cartil.15, 1178–1189 (2007).
Shirasawa, S. et al. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J. Cell. Biochem.97, 84–97 (2006).
Miyamoto, C., Matsumoto, T., Sakimura, K. & Shindo, H. Osteogenic protein-1 with transforming growth factor-beta1: potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J. Orthop. Sci.12, 555–561 (2007).
Koizumi, K. et al. Synovial mesenchymal stem cells from osteo- or rheumatoid arthritis joints exhibit good potential for cartilage repair using a scaffold-free tissue engineering approach. Osteoarthr. Cartil.24, 1413–1422 (2016).
Enomoto, T. et al. Timing of intra-articular injection of synovial mesenchymal stem cells affects cartilage restoration in a partial thickness cartilage defect model in rats. Cartilage11, 122–129 (2020).
Mak, J. et al. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci. Rep.6, 23076 (2016).
Kondo, S. et al. Transplantation of aggregates of autologous synovial mesenchymal stem cells for treatment of cartilage defects in the femoral condyle and the femoral groove in microminipigs. Am. J. Sports Med.47, 2338–2347 (2019).
Ozeki, N. et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil.24, 1061–1070 (2016).
Zhu, Y. et al. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther.8, 64 (2017).
Guo, S. C. et al. Exosomes from human synovial-derived mesenchymal stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head in the rat. Int. J. Biol. Sci.12, 1262–1272 (2016).
Kim, Y. S. & Koh, Y. G. Comparative matched-pair analysis of open-wedge high tibial osteotomy with versus without an injection of adipose-derived mesenchymal stem cells for varus knee osteoarthritis: clinical and second-look arthroscopic results. Am. J. Sports Med.46, 2669–2677 (2018).
Manferdini, C. et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum.65, 1271–1281 (2013).
Skalska, U. & Kontny, E. Adipose-derived mesenchymal stem cells from infrapatellar fat pad of patients with rheumatoid arthritis and osteoarthritis have comparable immunomodulatory properties. Autoimmunity49, 124–131 (2016).
Damia, E. et al. Adipose-derived mesenchymal stem cells: are they a good therapeutic strategy for osteoarthritis? Int. J. Mol. Sci.19, 1926 (2018).
Maumus, M. et al. Adipose mesenchymal stem cells protect chondrocytes from degeneration associated with osteoarthritis. Stem Cell Res.11, 834–844 (2013).
Lee, W. S., Kim, H. J., Kim, K. I., Kim, G. B. & Jin, W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl. Med.8, 504–511 (2019).
Tofino-Vian, M., Guillen, M. I., Perez Del Caz, M. D., Castejon, M. A. & Alcaraz, M. J. Extracellular vesicles from adipose-derived mesenchymal stem cells downregulate senescence features in osteoarthritic osteoblasts. Oxid. Med. Cell. Longev.2017, 7197598 (2017).
Tofino-Vian, M., Guillen, M. I., Perez Del Caz, M. D., Silvestre, A. & Alcaraz, M. J. Microvesicles from human adipose tissue-derived mesenchymal stem cells as a new protective strategy in osteoarthritic chondrocytes. Cell. Physiol. Biochem.47, 11–25 (2018).
Woo, C. H. et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles.9, 1735249 (2020).
Zhao, C. et al. Exosomes from adiposederived stem cells promote chondrogenesis and suppress inflammation by upregulating miR145 and miR221. Mol. Med. Rep.21, 1881–1889 (2020).
Ma, Q., Liao, J. & Cai, X. Different sources of stem cells and their application in cartilage tissue engineering. Curr. Stem Cell Res. Ther.13, 568–575 (2018).
Oldershaw, R. A. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int. J. Exp. Pathol.93, 389–400 (2012).
Hwang, N. S. & Elisseeff, J. Application of stem cells for articular cartilage regeneration. J. Knee Surg.22, 60–71 (2009).
Mamidi, M. K., Das, A. K., Zakaria, Z. & Bhonde, R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthr. Cartil.24, 1307–1316 (2016).
Gibson, J. D. et al. Regeneration of articular cartilage by human ESC-derived mesenchymal progenitors treated sequentially with BMP-2 and Wnt5a. Stem Cells Transl. Med.6, 40–50 (2017).
Zhang, S. et al. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil.24, 2135–2140 (2016).
Zhang, S. et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials156, 16–27 (2018).
Wang, Y. et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther.8, 189 (2017).
Zhang, S. et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials200, 35–47 (2019).
Wang, R., Xu, B. & Xu, H. TGF-beta1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle17, 2756–2765 (2018).
Zavatti, M., Beretti, F., Casciaro, F., Bertucci, E. & Maraldi, T. Comparison of the therapeutic effect of amniotic fluid stem cells and their exosomes on monoiodoacetate-induced animal model of osteoarthritis. BioFactors46, 106–117 (2019).
Yan, L. & Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol.36, 165–178 (2019).
Luo, P., Jiang, C., Ji, P., Wang, M. & Xu, J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther.10, 216 (2019).
Tai, Y. L., Chen, K. C., Hsieh, J. T. & Shen, T. L. Exosomes in cancer development and clinical applications. Cancer Sci.109, 2364–2374 (2018).
Deng, H. et al. Lipid, protein, and microRNA composition within mesenchymal stem cell-derived exosomes. Cell. Reprogramming.20, 178–186 (2018).
Zhang, Z. G., Buller, B. & Chopp, M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol.15, 193–203 (2019).
Ragni, E. et al. Identification of miRNA reference genes in extracellular vesicles from adipose derived mesenchymal stem cells for studying osteoarthritis. Int. J. Mol. Sci.20, 1108 (2019).
Carames, B. et al. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis.71, 575–581 (2012).
Takayama, K. et al. Local intra-articular injection of rapamycin delays articular cartilage degeneration in a murine model of osteoarthritis. Arthritis Res. Ther.16, 482 (2014).
Zhang, Y. et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann. Rheum. Dis.74, 1432–1440 (2015).
Dragomir, M., Chen, B. & Calin, G. A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res.7, S243–S252 (2018).
Liu, T. et al. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget7, 85551–85563 (2016).
Isin, M. et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front. Genet.6, 168 (2015).
Conigliaro, A. et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer14, 155 (2015).
Lang, H. L. et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci.21, 959–972 (2017).
Zheng, R. et al. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol. Cancer17, 143 (2018).
Patel, N. A. et al. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflammation.15, 204 (2018).
Liu, Y. et al. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem. J.475, 3629–3638 (2018).
Liu, Y. et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle17, 2411–2422 (2018).
Gross, J. C., Chaudhary, V., Bartscherer, K. & Boutros, M. Active Wnt proteins are secreted on exosomes. Nat. Cell Biol.14, 1036–1045 (2012).
Buschow, S. I., Liefhebber, J. M., Wubbolts, R. & Stoorvogel, W. Exosomes contain ubiquitinated proteins. Blood Cells Mol. Dis.35, 398–403 (2005).
Thery, C. et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol.147, 599–610 (1999).
Yuan, D. et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials142, 1–12 (2017).
Whiteside, T. L. Exosomes carrying immunoinhibitory proteins and their role in cancer. Clin. Exp. Immunol.189, 259–267 (2017).
Goetzl, E. J. et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J.30, 4141–4148 (2016).
Tsuno, H. et al. A proteomic analysis of serum-derived exosomes in rheumatoid arthritis. BMC Rheumatol.2, 35 (2018).
Kawakami, K. et al. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer17, 316 (2017).
Rabinowits, G., Gercel-Taylor, C., Day, J. M., Taylor, D. D. & Kloecker, G. H. Exosomal microRNA: a diagnostic marker for lung cancer. Clin. Lung Cancer10, 42–46 (2009).
Lv, L. L. et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am. J. Physiol. Ren. Physiol.305, F1220–F1227 (2013).
Song, J. et al. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin. Exp. Med.15, 121–126 (2015).
van Spil, W. E., DeGroot, J., Lems, W. F., Oostveen, J. C. & Lafeber, F. P. Serum and urinary biochemical markers for knee and hip-osteoarthritis: a systematic review applying the consensus BIPED criteria. Osteoarthr. Cartil.18, 605–612 (2010).
Charni, N., Juillet, F. & Garnero, P. Urinary type II collagen helical peptide (HELIX-II) as a new biochemical marker of cartilage degradation in patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum.52, 1081–1090 (2005).
Garnero, P., Charni, N., Juillet, F., Conrozier, T. & Vignon, E. Increased urinary type II collagen helical and C telopeptide levels are independently associated with a rapidly destructive hip osteoarthritis. Ann. Rheum. Dis.65, 1639–1644 (2006).
van der Lubbe, N. et al. The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension60, 741–748 (2012).
Yazarlou, F. et al. Urinary exosomal expression of long non-coding RNAs as diagnostic marker in bladder cancer. Cancer Manag. Res.10, 6357–6365 (2018).
Street, J. M., Koritzinsky, E. H., Glispie, D. M., Star, R. A. & Yuen, P. S. Urine exosomes: an emerging trove of biomarkers. Adv. Clin. Chem.78, 103–122 (2017).
Rashed, M. H. et al. Exosomes: from garbage bins to promising therapeutic targets. Int. J. Mol. Sci.18, 538 (2017).
Gernapudi, R. et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat.150, 685–695 (2015).
Hornick, N. I. et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci. Signal.9, ra88 (2016).
Chen, P. et al. Extraction and identification of synovial tissue-derived exosomes by different separation techniques. J. Orthop. Surg. Res.15, 97 (2020).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature546, 498–503 (2017).
Qi, H. et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano.10, 3323–3333 (2016).
Tian, T. et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials150, 137–149 (2018).
Gilligan K. E., Dwyer R. M. Engineering exosomes for cancer therapy. Int. J. Mol. Sci. 18, 1122 (2017).
Liu, X. et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale9, 4430–4438 (2017).
Wang, C. et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics9, 65–76 (2019).
Shi, Q. et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front. Physiol.8, 904 (2017).
Han, C. et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci.7, 2920–2933 (2019).
Liu, X. et al. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/beta-catenin signaling pathway. J. Orthop. Surg. Res.14, 470 (2019).
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0800802); the National Natural Science Foundation of China (No. 81530071; No. 31571382; No. 81871817); the Key Project of Innovation Program in Military Medicine (16CXZ016); the Sports Scientific Research Project of Chongqing (B201801); the Basic and Advanced Research Project in Chongqing (cstc2017jcyjAX0148); and the Project of the State Key Laboratory of Trauma, Burns and Combined Injury (No. SKLZZ(III)201601).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ni, Z., Zhou, S., Li, S. et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res 8, 25 (2020). https://doi.org/10.1038/s41413-020-0100-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41413-020-0100-9
This article is cited by
-
Natural products in osteoarthritis treatment: bridging basic research to clinical applications
Chinese Medicine (2024)
-
Therapeutic and immunomodulatory potentials of mesenchymal stromal/stem cells and immune checkpoints related molecules
Biomarker Research (2024)
-
Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies
Breast Cancer Research (2024)
-
Non-bone-derived exosomes: a new perspective on regulators of bone homeostasis
Cell Communication and Signaling (2024)
-
M2 macrophage-derived exosomal miR-26b-5p regulates macrophage polarization and chondrocyte hypertrophy by targeting TLR3 and COL10A1 to alleviate osteoarthritis
Journal of Nanobiotechnology (2024)