Abstract
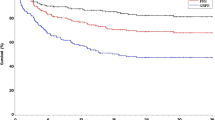
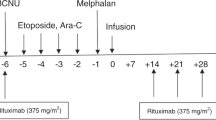
Curative potential of allogeneic transplantation (AlloSCT) in high-risk non-Hodgkin lymphoma (NHL) could be enhanced by the integration of Ofatumumab (OFA), a 2nd generation anti-CD20 moAb, due to an antitumor effect and a role over graft-versus-host disease (GVHD). In this phase II trial (NCT01613300), we investigated safety and effectiveness of OFA-based reduced intensity conditioning (RIC). High-risk B-cell NHL patients with chemorrefractory disease or post-autologous SCT relapse were eligible. OFA was added to a standard RIC regimen. Primary endpoint was grade 3-4 aGVHD rate, while secondary endpoints included CR and survival rates. Thirty-three patients were included (median age 51; diffuse large B-cell:68%, HLA-identical donor: 74%). No grade >2 OFA toxicity was observed. Acute GVHD affected 77% of patients (16% grade 3-4). Remarkably, GVHD achieved CR in 75% of patients after first-line treatment. Chronic GVHD, primarily mild or moderate, occurred in 54% of patients. NHL CR rate at day +100 was 81%. Relapses occurred in 7 patients after a median of 3 months. Causes of death were lymphoma progression (5), infections (10), and GVHD (2). At 24 months, progression-free and overall survival rates were 50.1 and 51.6% respectively. OFA-RIC regimen is safe and effective, though acute GVHD remains a significant complication. However, data suggest that OFA could mitigate its severity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, de Witte T, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transpl. 2003;31:667–78.
Rezvani AR, Storer B, Maris M, Sorror ML, Agura E, Maziarz RT, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:211–7.
Khouri IF, Champlin RE. Nonmyeloablative allogeneic stem cell transplantation for non-hodgkin lymphoma. Cancer J. 2012;18:457–62.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl J Med. 2019;380:45–56.
Ratanatharathorn V, Logan B, Wang D, Horowitz M, Uberti JP, Ringden O, et al. Prior rituximab correlates with less acute graft-versus-host disease and better survival in B-cell lymphoma patients who received allogeneic peripheral blood stem cell transplantation. Br J Haematol. 2009;145:816–24.
Crocchiolo R, Castagna L, El-Cheikh J, Helvig A, Fürst S, Faucher C, et al. Prior rituximab administration is associated with reduced rate of acute GVHD after in vivo T-cell depleted transplantation in lymphoma patients. Exp Hematol. 2011;39:892–6.
Arai S, Sahaf B, Narasimhan B, Chen GL, Jones CD, Lowsky R, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–54.
Cutler C, Kim HT, Bindra B, Sarantopoulos S, Ho VT, Chen YB, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–7.
Dodero A, Patriarca F, Milone G, Sarina B, Miceli R, Iori A, et al. Allogeneic Stem Cell Transplantation for Relapsed/Refractory B Cell Lymphomas: Results of a Multicenter Phase II Prospective Trial including Rituximab in the Reduced-Intensity Conditioning Regimen. Biol Blood Marrow Transpl. 2017;23:1102–9. https://pubmed.ncbi.nlm.nih.gov/28390983/.
Ruan Y, Chen L, Luo T, Xie D, Cao W, Liu X, et al. Applying rituximab during the conditioning regimen prevents Epstein-Barr virus infection following allogeneic hematopoietic stem cell transplant in a children’s cohort: a retrospective case-control study. Infect Dis Ther. 2023. https://pubmed.ncbi.nlm.nih.gov/37470925/
Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Filì C, et al. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transpl. 2007;40:273–7.
Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JGJ, Parren PWHI, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than by RTX. J Immunol. 2009;183:749–58.
Barth MJ, Hernandez-Ilizaliturri FJ, Mavis C, Tsai PC, Gibbs JF, Deeb G, et al. Ofatumumab demonstrates activity against rituximab-sensitive and -resistant cell lines, lymphoma xenografts and primary tumour cells from patients with B-cell lymphoma. Br J Haematol. 2012;156:490–8.
Karlin L, Coiffier B. Ofatumumab in the treatment of non-Hodgkin’s lymphomas. Expert Opin Biol Ther. 2015;15:1085–91.
Perez-Simón JA, Martino R, Parody R, Cabrero M, Lopez-Corral L, Valcarcel D, et al. The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica. 2013;98:526–32.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15:825–8.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21:389–401.e1.
Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–9.
A’Hern RP. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–66.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143:395–403. https://pubmed.ncbi.nlm.nih.gov/18759762/.
van Kampen RJW, Canals C, Schouten HC, Nagler A, Thomson KJ, Vernant JP, et al. Allogeneic stem-cell transplantation as salvage therapy for patients with diffuse large B-cell non-Hodgkin’s lymphoma relapsing after an autologous stem-cell transplantation: an analysis of the European Group for Blood and Marrow Transplantation Registry. J Clin Oncol. 2011;29:1342–8.
Dreger P, Sureda A, Ahn KW, Eapen M, Litovich C, Finel H, et al. PTCy-based haploidentical vs matched related or unrelated donor reduced-intensity conditioning transplant for DLBCL. Blood Adv. 2019;3:360–9.
Salhotra A, Mei M, Stiller T, Mokhtari S, Herrera AF, Chen R, et al. Outcomes of patients with recurrent and refractory lymphoma undergoing allogeneic hematopoietic cell transplantation with BEAM conditioning and sirolimus- and tacrolimus-based GVHD prophylaxis. Biol Blood Marrow Transpl. 2019;25:287–92. https://pubmed.ncbi.nlm.nih.gov/30227232/.
Robinson SP, Goldstone AH, Mackinnon S, Carella A, Russell N, De Elvira CR, et al. Chemoresistant or aggressive lymphoma predicts for a poor outcome following reduced-intensity allogeneic progenitor cell transplantation: an analysis from the Lymphoma Working Party of the European Group for Blood and Bone Marrow Transplantation. Blood. 2002;100:4310–6. https://pubmed.ncbi.nlm.nih.gov/12393626/.
Stamouli M, Gkirkas K, Karagiannidi A, Iliakis T, Chondropoulos S, Thomopoulos T, et al. Allogeneic stem cell transplantation with a novel reduced intensity conditioning regimen for the treatment of patients with primary cutaneous T-cell lymphomas. Clin Hematol Int. 2021;3:72. https://pubmed.ncbi.nlm.nih.gov/34595469/.
Author information
Authors and Affiliations
Consortia
Contributions
MC authored the manuscript; DC was the principal investigator of the trial and supervised manuscript preparation overseeing all the critical aspects; all remaining authors contributed to trial design, patient recruitment and monitoring, and manuscript review. All authors approve the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cabrero, M., López-Corral, L., Jarque, I. et al. Ofatumumab as part of reduced intensity conditioning in high risk B-cell lymphoma patients: final long-term analysis from a prospective multicenter Phase-II Trial. Bone Marrow Transplant 59, 359–365 (2024). https://doi.org/10.1038/s41409-023-02171-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02171-5