Abstract
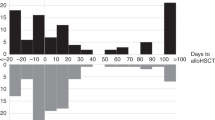
Clostridioides difficile infection (CDI) is common after allogeneic hematopoietic cell transplantation (alloHCT). The determination of incidence, risk factors, and impact of CDI on alloHCT outcomes is an unmet need. The study examines all patients aged 2 years and older who received first alloHCT for acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), or myelodysplastic syndrome (MDS) between 2013 and 2018 at US centers and reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) data registry. In total, 826 patients with CDI and 6723 controls from 127 centers were analyzed. The cumulative incidence of CDI by day 100 was 18.7% (99% CI: 15–22.7%) and 10.2% (99% CI: 9.2–11.1%) in pediatric and adult patients, respectively, with a median time to diagnosis at day +13. CDI was associated with inferior overall survival (OS) (p = 0.0018) and a 2.58-fold [99% CI: 1.43–4.66; p < 0.001] increase in infection-related mortality (IRM). There was a significant overlap in the onset of acute graft versus host disease (aGVHD) and CDI. IRM increased to >4 fold when CDI + aGVHD was considered. Despite advances in the management of CDI, increased IRM and decreased OS still results from CDI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
References
Dubberke ER, Reske KA, Olsen MA, Bommarito KM, Seiler S, Silveira FP, et al. Risk for Clostridium difficile infection after allogeneic hematopoietic cell transplant remains elevated in the postengraftment period. Transpl Direct. 2017;3:e145.
Obeid KM, Sapkota S, Cao Q, Richmond S, Watson AP, Karadag FK, et al. Early Clostridioides difficile infection characterizations, risks, and outcomes in allogeneic hematopoietic stem cell and solid organ transplant recipients. Transpl Infect Dis. 2022;24:e13720.
Alonso CD, Marr KA. Clostridium difficile infection among hematopoietic stem cell transplant recipients: beyond colitis. Curr Opin Infect Dis. 2013;26:326–31.
Alonso CD, Treadway SB, Hanna DB, Huff CA, Neofytos D, Carroll KC, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2012;54:1053–63.
Bhutani D, Jaiyeoba C, Kim S, Naylor P, Uberti JP, Ratanatharathorn V, et al. Relationship between clostridium difficile infection and gastrointestinal graft versus host disease in recipients of allogeneic stem cell transplantation. Bone Marrow Transpl. 2019;54:164–7.
Willems L, Porcher R, Lafaurie M, Casin I, Robin M, Xhaard A, et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: incidence, risk factors, and outcome. Biol Blood Marrow Transpl. 2012;18:1295–301.
Mayer EF, Maron G, Dallas RH, Ferrolino J, Tang L, Sun Y, et al. A multicenter study to define the epidemiology and outcomes of Clostridioides difficile infection in pediatric hematopoietic cell and solid organ transplant recipients. Am J Transpl. 2020;20:2133–42.
Willis DN, Huang FS, Elward AM, Wu N, Magnusen B, Dubberke ER, et al. Clostridioides difficile Infections in Inpatient Pediatric Oncology Patients: A Cohort Study Evaluating Risk Factors and Associated Outcomes. J Pediatr Infect Dis Soc. 2021;10:302–8.
Barbar R, Hayden R, Sun Y, Tang L, Hakim H. Epidemiologic and Clinical Characteristics of Clostridioides difficile Infections in Hospitalized and Outpatient Pediatric Oncology and Hematopoietic Stem Cell Transplant Patients. Pediatr Infect Dis J. 2021;40:655–62.
Amberge S, Kramer M, Schrottner P, Heidrich K, Schmelz R, Middeke JM, et al. Clostridium Difficile infections in patients with AML or MDS undergoing allogeneic hematopoietic stem cell transplantation identify high risk for adverse outcome. Bone Marrow Transpl. 2020;55:367–75.
Chakrabarti S, Lees A, Jones SG, Milligan DW. Clostridium difficile infection in allogeneic stem cell transplant recipients is associated with severe graft-versus-host disease and non-relapse mortality. Bone Marrow Transpl. 2000;26:871–6.
Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61.
Kim S, Logan B, Riches M, Chen M, Ahn KW. Statistical Methods for Time-Dependent Variables in Hematopoietic Cell Transplantation Studies. Transpl Cell Ther. 2021;27:125–32.
Commenges D, Andersen PK. Score test of homogeneity for survival data. Lifetime Data Anal. 1995;1:145–56. discussion 57-9
Boyle NM, Magaret A, Stednick Z, Morrison A, Butler-Wu S, Zerr D, et al. Evaluating risk factors for Clostridium difficile infection in adult and pediatric hematopoietic cell transplant recipients. Antimicrob Resist Infect Control. 2015;4:41.
Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl J Med. 2000;342:390–7.
Jung Ok S. Clostridium difficile in children: To treat or not to treat. Pediatr Gastroenterol Hepatol Nutr. 2014;17:80–4.
Shah NN, McClellan W, Flowers CR, Lonial S, Khoury H, Waller EK, et al. Evaluating Risk Factors for Clostridium difficile Infection In Stem Cell Transplant Recipients: A National Study. Infect Control Hosp Epidemiol. 2017;38:651–7.
Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–11.
Salamonowicz M, Ociepa T, Fraczkiewicz J, Szmydki-Baran A, Matysiak M, Cyzzewski K, et al. Incidence, course, and outcome of Clostridium difficile infection in children with hematological malignancies or undergoing hematopoietic stem cell transplantation. Eur J Clin Microbiol Infect Dis. 2018;37:1805–12.
Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, et al. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–92.
Staffas A, Burgos da Silva M, van den Brink MR. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017;129:927–33.
van Lier YF, Davids M, Haverkate NJE, de Groot PF, Donker ML, Meijer E, et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med. 2020;12:eaaz8926.
Murphy S, Nguyen VH. Role of gut microbiota in graft-versus-host disease. Leuk Lymphoma. 2011;52:1844–56.
Dubberke ER, Reske KA, Olsen MA, Bommarito K, Cleveland AA, Silveira FP, et al. Epidemiology and outcomes of Clostridium difficile infection in allogeneic hematopoietic cell and lung transplant recipients. Transpl Infect Dis. 2018;20:e12855.
Lavallee C, Labbe AC, Talbot JD, Alonso CD, Marr KA, Cohen S, et al. Risk factors for the development of Clostridium difficile infection in adult allogeneic hematopoietic stem cell transplant recipients: a single-center study in Quebec, Canada. Transpl Infect Dis. 2017;19. https://doi.org/10.1111/tid.12648.
Morrisette T, Van Matre AG, Miller MA, Mueller SW, Bajrovic V, Abidi MZ, et al. Oral Vancomycin Prophylaxis as Secondary Prevention Against Clostridioides difficile Infection in the Hematopoietic Stem Cell Transplantation and Hematologic Malignancy Population. Biol Blood Marrow Transpl. 2019;25:2091–7.
Mullane KM, Winston DJ, Nooka A, Morris MI, Stiff P, Dugan MJ, et al. A Randomized, Placebo-controlled Trial of Fidaxomicin for Prophylaxis of Clostridium difficile-associated Diarrhea in Adults Undergoing Hematopoietic Stem Cell Transplantation. Clin Infect Dis. 2019;68:196–203.
Acknowledgements
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014–20–1–2832 and N00014–21–1–2954 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals, Inc.; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; Allovir, Inc.; Amgen, Inc.; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx Inc.; CRISPR; CSL Behring; CytoSen Therapeutics, Inc.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon, a Sanofi Company; Karius; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medac GmbH; Medexus Pharma; Merck & Co.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC, An AbbVie Company; Pluristem; PPD Development, LP; Sanofi; Sobi, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc.; Xenikos BV.
Author information
Authors and Affiliations
Contributions
MRamanathan and CU were responsible for designing the observational study, writing the paper, interpreting results, and updating references. SK, NH, and MC were responsible for extracting and analyzing data and creating figures and tables. PH, MBA, SJR, KMW, HML, BW, and DEY provided feedback for the paper. CGK, MAP, RFC, CD, and MRiches contributed to study design, writing the paper, interpreting results and updating references.
Corresponding author
Ethics declarations
Competing interests
Dr KMW reports having a R01 with NHLBI on biomarkers of engraftment; a TRP grant with Leukemia Lymphoma Society to develop a novel immunotherapy for AML; and having received payments from Rising Tide, NIH, and LLS for grant reviews. Dr MAP reports honoraria from Abbvie, Astellas, Bristol-Myers Squibb, Celgene, Equilium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, OrcaBio, Takeda, and VectivBio AG, Vor Biopharma; serves on DSMBs for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune; has ownership interests in NexImmune and Omerosl; has received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis; received payments from Adboards/Consulting for Novartis, Merck, Omeros, Incyte. Relationships: VP, ASTCT. Dr RFC reports as a consultant and/or Advisor for Ansun BioPharma, Janssen Pharmaceuticals, Inc., Merck & Co., Inc., Oxford Immunotec USA, Inc., Pulmotect, Inc., Takeda, Genentech, Karius, ADMA Biologics, Molecular Partners, and Xenex Laboratories; having received grant/research support from Ansun BioPharma, Eurofins-Viracor, Inc., Merck & Co., Inc., Takeda; Karius, Inc., Oxford Immunotec USA, Inc., and Genentech. Dr CED has received honorarium from Omeros and Alexion Pharmaceuticals. Dr MRiches reports IQVIA Biotech, employee (as of 1/10/22); Jazz Pharmaceuticals (research funding to university- end 1/10/22); Atara Bio-Pharma (research funding to university- end 1/10/22).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramanathan, M., Kim, S., He, N. et al. The incidence and impact of clostridioides difficile infection on transplant outcomes in acute leukemia and MDS after allogeneic hematopoietic cell transplant—a CIBMTR study. Bone Marrow Transplant 58, 360–366 (2023). https://doi.org/10.1038/s41409-022-01896-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01896-z