Abstract
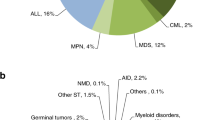
A total of 5642 hematopoietic cell transplants (HCT) in 5445 patients (2196—40% allogeneic and 3249—60% autologous) were reported by 127 teams in 14 Latin American countries that answered the 2018 LABMT/WBMT Global Transplant Activity survey. The transplant rate (defined as the number of first transplants per 10 million inhabitants per year) was 85 (51 autologous and 34 allogeneic) in 2018. The main indications for allogeneic HCT were acute leukemias (60%), while plasma cell disorders and lymphomas were the most common conditions warranting autologous HCT (50 and 36%, respectively). In the allogeneic HCT, HLA-identical siblings were the main type of donor (44%) followed by related mismatched/haploidentical donors (32%). Peripheral blood stem cells were used in 98% of the autologous and in 64% of the allogeneic transplants. From 2012 to 2018, there was a 64% increase of reported HCT (54% in autologous and 80% in allogeneic). In the allogeneic setting, the most pronounced increase in donor type was observed in haploidentical relatives (from 94 procedures in 2012 up to 710 in 2018), surpassing unrelated donors as of 2017. Significant trends detected in Latin America include rising numbers of the procedures reported, a faster increase in allogeneic HCT compared with autologous HCT and a significant increase in family mismatched/haploidentical donors. The LABMT/WBMT activity survey provides useful data to understand the HCT activity and trends in Latin America.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26.
WBMT. https://www.wbmt.org/. 22 Oct 2021.
Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol. 2015;2:e91–e100.
Latin American Bone Marrow Transplantation Group - LABMT. https://www.wbmt.org/member-societies-of-wbmt/labmt/. Accessed 22 Oct 2021.
Jaimovich G, Martinez Rolon J, Baldomero H, Rivas M, Hanesman I, Bouzas L, et al. Latin America: The next region for haematopoietic transplant progress. Bone Marrow Transplant. 2017;52:671–7.
DISMCLFD List of Disease Classifications | EBMT. https://www.ebmt.org/ebmt/documents/dismclfd-list-disease-classifications. Accessed 27 Sep 2021.
World Population Prospects - Population Division - United Nations. https://population.un.org/wpp/Download/Standard/Population/. Accessed 27 Sep 2021.
WHO Regional offices. https://www.who.int/about/who-we-are/regional-offices. Accessed 27 Sep 2021.
Niederwieser D, Baldomero H, Szer J, Gratwohl M, Aljurf M, Atsuta Y, et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016;51:778–85.
Baldomero H, Aljurf M, Zaidi SZA, Hashmi SK, Ghavamzadeh A, Elhaddad A, et al. Narrowing the gap for hematopoietic stem cell transplantation in the East-Mediterranean/African region: comparison with global HSCT indications and trends. Bone Marrow Transplant. 2019;54:402–17.
Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2021. https://doi.org/10.3324/haematol.279189
Iida M, Dodds A, Akter M, Srivastava A, Moon JH, Dung PC, et al. The 2016 APBMT Activity Survey Report: trends in haploidentical and cord blood transplantation in the Asia-Pacific region. Blood Cell Ther. 2021;4:20–28.
Cowan AJ, Baldomero H, Atsuta Y, Mikhael J, Aljurf M, Seber A, et al. The Global State of Hematopoietic Cell Transplantation for Multiple Myeloma: an analysis of the worldwide network of Blood and Marrow Transplantation database and the global burden of disease study. Biol Blood Marrow Transplant. 2020;26:2372–7.
Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transplant. 2020;55:1604–13.
Gale RP, Seber A, Bonfim C, Pasquini M. Haematopoietic cell transplants in Latin America. Bone Marrow Transplant. 2016;51:898–905.
Jaimovich G, Gale RP, Hanesman I, Vazquez A, Hammerschlak N, Simoes BP, et al. The paradox of haematopoietic cell transplant in Latin America. Bone Marrow Transplant. 2021;56:2382–8.
Shaw BE. Related haploidentical donors are a better choice than matched unrelated donors: Counterpoint. Blood Adv. 2017;1:401–6.
Fuchs EJ. Related haploidentical donors are a better choice than matched unrelated donors: point. Blood Adv. 2017;1:397–400.
Basquiera AL, Berro M, Yantorno S, Castro M, Requejo A, Sorrentino M, et al. Haploidentical transplant in adult patients with acute lymphoblastic leukemia in Argentina: a comparison with matched related and unrelated donors. Bone Marrow Transplant. 2020;55:400–8.
Bonfim C, Ribeiro L, Nichele S, Loth G, Bitencourt M, Koliski A, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide for children and adolescents with fanconi anemia. Biol Blood Marrow Transplant. 2017;23:310–7.
Fernandes JF, Nichele S, Arcuri LJ, Ribeiro L, Zamperlini-Netto G, Loth G, et al. Outcomes after haploidentical stem cell transplantation with post-transplantation cyclophosphamide in patients with primary immunodeficiency diseases. Biol Blood Marrow Transplant. 2020;26:1923–9.
Trujillo ÁM, Karduss AJ, Suarez G, Pérez R, Ruiz G, Cardona A, et al. Haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide in children with high-risk leukemia using a reduced-intensity conditioning regimen and peripheral blood as the stem cell source. Transplant Cell Ther. 2021;27:427.e1–427.e7.
Sarmiento M, Ramirez P, Jara V, Bertin P, Galleguillos M, Rodriguez I, et al. Haploidentical transplantation outcomes are comparable with those obtained with identical human leukocyte antigen allogeneic transplantation in Chilean patients with benign and malignant hemopathies. Hematol Transfus Cell Ther. 2020;42:40–5.
Colunga-Pedraza PR, Gómez-De León A, Rodríguez-Roque CS, Morcos-Sandino M, Colunga-Pedraza JE, Cantú-Rodriguez OG, et al. Outpatient haploidentical stem cell transplantation using post-transplant cyclophosphamide is safe and feasible. Transplant Cell Ther. 2021;27:259.e1–259.e6.
Wong A, Moreno M, Castillo M, Navarro J, Aranda L. Haploidentical Bmt and post transplant cyclophosphamide: first peruvian experience. Biol Blood Marrow Transplant. 2018;24:S353.
Dufort G, Castillo L, Pisano S, Castiglioni M, Carolina P, Andrea I, et al. Haploidentical hematopoietic stem cell transplantation in children with high-risk hematologic malignancies: outcomes with two different strategies for GvHD prevention. Ex vivo T-cell depletion and post-transplant cyclophosphamide: 10 years of experience at a single center. Bone Marrow Transplant. 2016;51:1354–60.
Passweg JR, Baldomero H, Chabannon C, Basak GW, de la Cámara R, Corbacioglu S, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021;56:1651–64.
D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–e182.
Xu LP, Lu PH, Wu DP, Sun ZM, Liu QF, Han MZ, et al. Hematopoietic stem cell transplantation activity in China 2019: a report from the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant. 2021;56:2940–7.
Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–48.
Arrieta-Bolaños E, Oliveira DC, Barquera R. Differential admixture, human leukocyte antigen diversity, and hematopoietic cell transplantation in Latin America: challenges and opportunities. Bone Marrow Transplant. 2020;55:496–504.
WMDA worldwide organizations. https://share.wmda.info/display/WMDAREG/Database. Accessed 27 Sep 2021.
Aljurf M, Weisdorf D, Alfraih F, Szer J, Müller C, Confer D, et al. “Worldwide Network for Blood & Marrow Transplantation (WBMT) special article, challenges facing emerging alternate donor registries.”. Bone Marrow Transplant. 2019;54:1179–88.
Snowden JA, McGrath E, Duarte RF, Saccardi R, Orchard K, Worel N, et al. JACIE accreditation for blood and marrow transplantation: past, present and future directions of an international model for healthcare quality improvement. Bone Marrow Transplant. 2017;52:1367–71.
Pasquini MC, Srivastava A, Ahmed SO, Aljurf M, Atsuta Y, Doleysh C, et al. Worldwide network for Blood and Marrow Transplantation recommendations for establishing a hematopoietic cell transplantation program, Part I: minimum requirements and beyond. Biol Blood Marrow Transplant. 2019;25:2322–9.
Acknowledgements
LABMT would like to acknowledge the contribution of Dr Francisco Ramírez Osío for the development of the group and the implementation of the survey in our region. List of participating centers: Argentina: H.Aleman, S.Allende, S.Anchorena, H.Austral, H.Britanico, CEMIC, CENHYT-S. Britanico, CETRAMOR, H.de Clinicas, Corporación Médica de San Martin, F.Favaloro, I.Alexander Fleming, FUNDALEU, H. JP Garrahan, H.Italiano, H.Italiano-La Plata, H.Italiano-San Justo; H.R.Madariaga, H.Privado de Cordoba, S.Sagrado Corazon, H.San Martin, H.Santisima Trinidad, Sor Maria Ludovica, H.R.Rossi, H.V.Vilela. Bolivia: Los Olivos, Instituto Boliviano de Onco-hematología. Brasil: H.Brasilia, I.de Cardiologia do Distrito Federal, H.Rio Grande, H.Universitario Walter Cantidio, H.Real Portugues, H.Angelina Caron, Centro de Pesquisa Oncológicas (CEPON), H.Erasto Gaertner, H.Da Crianca Santo Antonio, H.De Clinicas De Porto Alegre, H.Moinhos de Vento, H.Nossa Senhora das Gracas, H.Nossa Senhora do Pilar, H.Pequeno Principe, H.Universitario de Londrina, H.Uopeccan de Cascavel, H.de Clinicas da Universidade Federal do Parana, H.das Clinicas da Universidade Federal de Minas Gerais, H.Universitario da Universidade de Juiz de Fora, H.Santa Genoveva, Nucleo de Hematologia e Oncologia - Belo Horizonte.Santa Casa de Misericórdia de Belo Horizonte, Complexo Hospitlar de Niteroi, H.Naval Marcilio Dias, I.Nacional de Cancer (INCA), Universidade do Estado do Rio de Janeiro, H.9 de Julho, H.A.C.Camargo Cancer Center, Bio Sana’s Servicos Medicos, H.Beneficiência Portuguêsa de São José do Rio Preto, Barretos Cancer Hospital, F.Faculdade Regional de Medicina de Sao Jose do Rio Preto, H.Samaritano, H.Dr. Miguel Soeiro - Unimed Sorocaba, H.Amaral Carvalho, H.Israelita Albert Einstein, Grupo de Apoio ao Adolescente e à Criança com Câncer (GRAACC), I.Tratamento do Câncer Infantil (ITACI), H.Sirio Libanes, H.Leforte Liberdade, H.das Clinicas da Faculdade de Medicina da Universidade de São Paulo, Universidade Estadual de Campinas (UNICAMP), Universidade Estadual de Sao Paulo - Botucatu, H.Sao Camilo - Unidade Pompeia. Chile: C.Alemana, H.L. Calvo Mackenna, C.Davila, H.Del Salvador, C.Santa Maria. Colombia: I.Cancerología C.Las Américas, C.Foscal, C.de Marly, Centro Medico Imbanaco, H.de la Misericordia, H.Universitario San Ignacio. Costa Rica: H.CIMA, H.México, H.San Juan de Dios. Cuba: H.Hermanos Ameijeiras, H.CIMEQ, Instituto de Hematología e Inmunología, H.Lucia Iniguez, H.Arnaldo Milián. Ecuador: Solca-Guayaquil. MEXICO: ABC Observatorio, H.Civil de Guadalajara, H.Civil de Guadalajara “Fray Antonio Alcande”, H.F.Gómez, UMAE IMSS Puebla, H.“Jose Eleuterio Gonzalez”, I.Nacional de Cancerología, I.Nacional de Pediatría, H.Puerta de Hierro Norte, H. CMN Siglo XXI, UMAE G.González Garza, I.Salvador Zubiran, C.Estatal Oncología M.Dorantes Mesa, C.Mérida, H.Angeles Lomas, C.Ruiz, H.Teletón. PANAMA: Caja del Seguro Social, Hospital del Niño. Paraguay: I.Previsión Social, H.Clínicas. Peru: H.G.Almenara Irigoyen, I.Nacional de Enf. Neoplásicas, I.Nacional de Salud del Niño-San Borja, H.Regabliati. Uruguay: H.Británico, H.Maciel, F.Perez-Scremini, Servicio Médico Integral. Venezuela: H.de Clínicas, Ciudad Hospitalaria Dr.E.Techera. F.: Fundacion/Fundacao, H.: Hospital, C.:Clinica, I.:Instituto.
Author information
Authors and Affiliations
Consortia
Contributions
CC, OGR, and SG conceived the analysis and drafted the manuscript. CC, OGR, and HB were responsible for the data collection and assembly. ALB, RB, LA, BP, CR, MCH., CH, BM, AGD, NM, CF, LA, LD, MH, AS, AK, GJ, JMR, CB, HG, MK, MA, MI, WS, DN, and YA were responsible for the integrity of the data and gave scientific input.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Correa, C., Gonzalez-Ramella, O., Baldomero, H. et al. Increasing access to hematopoietic cell transplantation in Latin America: results of the 2018 LABMT activity survey and trends since 2012. Bone Marrow Transplant 57, 881–888 (2022). https://doi.org/10.1038/s41409-022-01630-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01630-9