Abstract
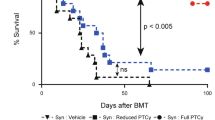
Allogeneic hematopoietic stem cell transplantation (allo-SCT) is a potentially curative therapy for FLT3 internal tandem duplication mutant (FLT3-ITD+) acute myeloid leukemia, but relapse rate is high. A recent study showed that sorafenib, a first generation FLT3 and multikinase inhibitor, enhanced graft-versus-leukemia (GVL) effects against FLT3-ITD+ leukemia via interleukin-15 (IL-15) production. However, it remains to be clarified whether this effect could be mediated by selective FLT3 inhibition. We investigated whether gilteritinib, a selective FLT3 inhibitor, could enhance GVL effects against FLT3-ITD transfected Ba/F3 leukemia (Ba/F3-FLT3-ITD) in mice. Oral administration of gilteritinib from day +5 to +14 after allo-SCT reduced expression of the co-inhibitory receptors PD-1 and TIGIT on donor CD8+ T cells and enhanced IL-15 expression in Ba/F3-FLT3-ITD. Bioluminescent imaging using luciferase-transfected Ba/F3-FLT3-ITD demonstrated that gilteritinib significantly suppressed leukemia expansion after allo-SCT, whereas it did not impact the morbidity or mortality of graft-versus-host disease (GVHD), resulting in significant improvement of overall survival. In conclusion, short-term administration of gilteritinib after allo-SCT enhanced GVL effects against FLT3-ITD+ leukemia without exacerbating GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–8. e-pub ahead of print.
Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv. 2020;4:1178–91. https://doi.org/10.1182/bloodadvances.2019000174. e-pub ahead of print.
Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–10. https://doi.org/10.1038/nature10738.
O’Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, et al. Acute myeloid leukemia, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:926–57. https://doi.org/10.6004/jnccn.2017.0116. e-pub ahead of print.
Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018;24:282–91. https://doi.org/10.1038/nm.4484. e-pub ahead of print.
Bornhauser M, Illmer T, Schaich M, Soucek S, Ehninger G, Thiede C, et al. Improved outcome after stem-cell transplantation in FLT3/ITD-positive AML. Blood. 2007;109:2264–5. https://doi.org/10.1182/blood-2006-09-047225. author reply 2265. e-pub ahead of print.
Canaani J, Labopin M, Huang XJ, Arcese W, Ciceri F, Blaise D, et al. T-cell replete haploidentical stem cell transplantation attenuates the prognostic impact of FLT3-ITD in acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Am J Hematol. 2018;93:736–44. https://doi.org/10.1002/ajh.25082. e-pub ahead of print.
Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30:735–41. https://doi.org/10.1200/JCO.2011.36.9868. e-pub ahead of print.
Schmid C, Labopin M, Socie G, Daguindau E, Volin L, Huynh A, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood. 2015;126:2062–9. https://doi.org/10.1182/blood-2015-06-651562. e-pub ahead of print.
DeZern AE, Sung A, Kim S, Smith BD, Karp JE, Gore SD, et al. Role of allogeneic transplantation for FLT3/ITD acute myeloid leukemia: outcomes from 133 consecutive newly diagnosed patients from a single institution. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2011;17:1404–9. https://doi.org/10.1016/j.bbmt.2011.02.003. e-pub ahead of print.
Didion JP, Buus RJ, Naghashfar Z, Threadgill DW, Morse HC 3rd, de Villena FP. SNP array profiling of mouse cell lines identifies their strains of origin and reveals cross-contamination and widespread aneuploidy. BMC Genomics. 2014;15:847 https://doi.org/10.1186/1471-2164-15-847. e-pub ahead of print.
Li S, Ilaria RL Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–412. https://doi.org/10.1084/jem.189.9.1399. e-pub ahead of print.
Naviaux RK, Costanzi E, Haas M, Verma IM. The pCL vector system: rapid production of helper-free, high-titer, recombinant retroviruses. J Virol. 1996;70:5701–5. https://doi.org/10.1128/JVI.70.8.5701-5705.1996. e-pub ahead of print.
Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J Jr, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–9.
Teshima T, Hill GR, Pan L, Brinson YS, van den Brink MR, Cooke KR, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. J Clin Investig. 1999;104:317–25. https://doi.org/10.1172/JCI7111
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244. e-pub ahead of print.
Burchert A. Maintenance therapy for FLT3-ITD-mutated acute myeloid leukemia. Haematologica. 2021;106:664–70. https://doi.org/10.3324/haematol.2019.240747. e-pub ahead of print.
Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26:2353–9. https://doi.org/10.1038/leu.2012.105. e-pub ahead of print.
Safaian NN, Czibere A, Bruns I, Fenk R, Reinecke P, Dienst A, et al. Sorafenib (Nexavar) induces molecular remission and regression of extramedullary disease in a patient with FLT3-ITD+ acute myeloid leukemia. Leuk Res. 2009;33:348–50. https://doi.org/10.1016/j.leukres.2008.04.017. e-pub ahead of print.
Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–71. https://doi.org/10.1182/blood-2009-03-208298. e-pub ahead of print.
Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Rollig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020: JCO1903345. https://doi.org/10.1200/JCO.19.03345. e-pub ahead of print.
Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J. et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020. https://doi.org/10.1016/S1470-2045(20)30455-1. e-pub ahead of print.
Uhl FM, Chen S, O’Sullivan D, Edwards-Hicks J, Richter G, Haring E, et al. Metabolic reprogramming of donor T cells enhances graft-versus-leukemia effects in mice and humans. Sci Transl Med. 2020;12. https://doi.org/10.1126/scitranslmed.abb8969. e-pub ahead of print.
Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2:1001–12. https://doi.org/10.1038/s42255-020-00280-9. e-pub ahead of print.
Asakura S, Hashimoto D, Takashima S, Sugiyama H, Maeda Y, Akashi K, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Investig. 2010;120:2370–8.
Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–7. https://doi.org/10.4049/jimmunol.171.3.1272. e-pub ahead of print.
Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. e-pub ahead of print.
Johnson BD, Truitt RL. Delayed infusion of immunocompetent donor cells after bone marrow transplantation breaks graft-host tolerance allows for persistent antileukemic reactivity without severe graft-versus-host disease. Blood. 1995;85:3302–12. e-pub ahead of print.
Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. https://doi.org/10.1038/nri1901. e-pub ahead of print.
Acknowledgements
We gratefully acknowledge Dr. Gerard Grosveld (Department of Genetics, St. Jude Children’s Research Hospital) for providing pMSCV-lus-IRES-YFP plasmid and Dr. Inder Verma (Addgene) for providing pCL-Eco.
Funding
This study was supported by JSPS KAKENHI (21K08409 to DH, 21390295 to TT, 20K17366 to HO, 21K16259 to TA, JP21H02775 to MN), Japan Society of Hematology Research Fund (TT), the Center of Innovation Program from JST (TT).
Author information
Authors and Affiliations
Contributions
DH and TT developed the conceptual framework of the study, designed the experiments, analyzed the data and wrote the paper. ZZ and YH conducted experiments, analyzed data, and wrote the paper. HS, RK, XC, KY, TS, TK, HT, TI, TA, and HO conducted experiments. MN supervised experiments.
Corresponding author
Ethics declarations
Competing interests
TT received a research grant from Kyowa Kirin, Chugai, Sanofi, Astellas, TEIJIN PHARMA, Fuji Pharma, NIPPON SHINYAKU, Personal Fees from Novartis, Merck, Kyowa Kirin, Takeda, Pfizer, Bristol-Myers Squibb, Non-Financial Support from Janssen, Novartis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Z., Hasegawa, Y., Hashimoto, D. et al. Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 57, 775–780 (2022). https://doi.org/10.1038/s41409-022-01619-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01619-4
This article is cited by
-
Overcoming relapse: prophylactic or pre-emptive use of azacitidine or FLT3 inhibitors after allogeneic transplantation for AML or MDS
International Journal of Hematology (2023)
-
FLT3-inhibitor therapy for prevention and treatment of relapse after allogeneic hematopoietic cell transplantation
International Journal of Hematology (2022)