Abstract
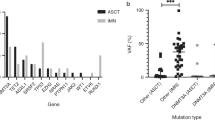
The role of WT1 protein in hematopoiesis and leukemogenesisis incompletely elucidated. WT1 overexpression is common in acute myeloid leukemia (AML); however, WT1 mutations occur in only about 10% of cases, with increasing incidence in the setting of relapse. In this study, we investigated the clinical and molecular characteristics of WT1 mutations in NPM1-mutated AML, to enhance our understanding of the biology and potential therapeutic implications of WT1 mutations. Our study cohort included 67 patients with NPM1 mutated AML and a median follow-up of 13.7 months. WT1 mutations were identified in 7% (n = 5) of patients at the time of initial diagnosis. WT1 mutant clones were presumed to be present as co-dominant clones in 3/5 and in subclonal populations in 2/5 cases based on variant allelic frequency (VAF) when compared with NPM1 mutation VAF. All WT1 mutations became undetectable at time of MRD-negative (NPM1-wild type) remission. None of these patients experienced relapse at the time of last follow-up (median, 15 months; range, 4.5–20.2 months). A total of 15/67 (22%) patients relapsed; among these patient, four (27%) relapsed with WT1 mutant AML. Three of four patients had undergone allogeneic hematopoietic stem cell transplantation (HSCT). None of these patients had detectable WT1 mutations at the time of initial diagnosis. WT1 mutations were presumed clonal in two cases and subclonal in the other two cases, based on VAF. Our results indicate that WT1 mutations contribute to relapse in NPM1 mutated AML, especially in the setting of HSCT. These findings suggest that emerging WT1 mutations may serve as a conduit for relapse in NPM1-mutated AML, and that sequential molecular profiling to evaluate potential emergent WT1 mutations during surveillance and particularly at relapse likely has prognostic value in patients with NPM1 mutated AML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maheswaran S, Park S, Bernard A, Morris JF, Rauscher FJ 3rd, Hill DE, et al. Physical and functional interaction between WT1 and p53 proteins. Proc Natl Acad Sci USA. 1993;90:5100–4.
Rong Y, Cheng L, Ning H, Zou J, Zhang Y, Xu F, et al. Wilms’ tumor 1 and signal transducers and activators of transcription 3 synergistically promote cell proliferation: a possible mechanism in sporadic Wilms’ tumor. Cancer Res. 2006;66:8049–57.
Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell 1990;60:509–20.
Rampal R, Alkalin A, Madzo J, Vasanthakumar A, Pronier E, Patel J, et al. DNA hydroxymethylation profiling reveals that WT1 mutations result in loss of TET2 function in acute myeloid leukemia. Cell Rep. 2014;9:1841–55.
Wang Y, Xiao M, Chen X, Chen L, Xu Y, Lv L, et al. WT1 recruits TET2 to regulate its target gene expression and suppress leukemia cell proliferation. Mol Cell. 2015;57:662–73.
Wilm B M-CR. The Role of WT1 in Embryonic Development and Normal Organ Homeostasis. n: Hastie N (eds) The Wilms’ Tumor (WT1) Gene Methods in Molecular Biology. 2016; 1467:Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-023-3_3.
Ariyaratana S, Loeb DM. The role of the Wilms tumour gene (WT1) in normal and malignant haematopoiesis. Expert Rev Mol Med. 2007;9:1–17.
Ellisen LW, Carlesso N, Cheng T, Scadden DT, Haber DA. The Wilms tumor suppressor WT1 directs stage-specific quiescence and differentiation of human hematopoietic progenitor cells. EMBO J. 2001;20:1897–909.
Loeb DM, Summers JL, Burwell EA, Korz D, Friedman AD, Sukumar S. An isoform of the Wilms’ tumor suppressor gene potentiates granulocytic differentiation. Leukemia 2003;17:965–71.
Vosberg S, Hartmann L, Metzeler KH, Konstandin NP, Schneider S, Varadharajan A, et al. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation is associated with gain of WT1 alterations and high mutation load. Haematologica 2018;103:e581–e4.
Rein LA, Chao NJ. WT1 vaccination in acute myeloid leukemia: new methods of implementing adoptive immunotherapy. Expert Opin Investig Drugs. 2014;23:417–26.
Hou HA, Huang TC, Lin LI, Liu CY, Chen CY, Chou WC, et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: stability during disease evolution and implication of its incorporation into a survival scoring system. Blood 2010;115:5222–31.
Ok CY, Loghavi S, Sui D, Wei P, Kanagal-Shamanna R, Yin CC, et al. Persistent IDH1/2 mutations in remission can predict relapse in patients with acute myeloid leukemia. Haematologica 2019;104:305–11.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47.
Loghavi S, DiNardo CD, Furudate K, Takahashi K, Tanaka T, Short NJ, et al. Flow cytometric immunophenotypic alterations of persistent clonal haematopoiesis in remission bone marrows of patients with NPM1-mutated acute myeloid leukaemia. Br J Haematol. 2021;192:1054–63.
Boublikova L, Kalinova M, Ryan J, Quinn F, O’Marcaigh A, Smith O, et al. Wilms’ tumor gene 1 (WT1) expression in childhood acute lymphoblastic leukemia: a wide range of WT1 expression levels, its impact on prognosis and minimal residual disease monitoring. Leukemia 2006;20:254–63.
Rezvani K, Yong AS, Savani BN, Mielke S, Keyvanfar K, Gostick E, et al. Graft-versus-leukemia effects associated with detectable Wilms tumor-1 specific T lymphocytes after allogeneic stem-cell transplantation for acute lymphoblastic leukemia. Blood 2007;110:1924–32.
Sugiyama H. WT1 (Wilms’ tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol. 2010;40:377–87.
Vasu S, Blum W. Emerging immunotherapies in older adults with acute myeloid leukemia. Curr Opin Hematol. 2013;20:107–14.
Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood 2000;95:286–93.
Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl J Med. 2009;361:1249–59.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl J Med. 2012;366:1079–89.
Summers K, Stevens J, Kakkas I, Smith M, Smith LL, Macdougall F, et al. Wilms’ tumour 1 mutations are associated with FLT3-ITD and failure of standard induction chemotherapy in patients with normal karyotype AML. Leukemia 2007;21:550–1. author reply 2
King-Underwood L, Pritchard-Jones K. Wilms’ tumor (WT1) gene mutations occur mainly in acute myeloid leukemia and may confer drug resistance. Blood 1998;91:2961–8.
Quek L, Ferguson P, Metzner M, Ahmed I, Kennedy A, Garnett C, et al. Mutational analysis of disease relapse in patients allografted for acute myeloid leukemia. Blood Adv. 2016;1:193–204.
Greif PA, Hartmann L, Vosberg S, Stief SM, Mattes R, Hellmann I, et al. Evolution of Cytogenetically Normal Acute Myeloid Leukemia During Therapy and Relapse: An Exome Sequencing Study of 50 Patients. Clin Cancer Res. 2018;24:1716–26.
Sala EBF, Biavasco F, Bucci G, Toffalori C, Biancolini D, Antonini E, et al. Mapping the evolution of the mutational landscape of acute myeloid leukemia from diagnosis to post-transplantation relapse and its interplay with immune evasion mechanisms. Blood. 2019;134.
Sugiyama H. Wilms’ tumor gene WT1: its oncogenic function and clinical application. Int J Hematol. 2001;73:177–87.
Doubrovina E, Carpenter T, Pankov D, Selvakumar A, Hasan A, O’Reilly RJ. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood 2012;120:1633–46.
Rezvani K, Grube M, Brenchley JM, Sconocchia G, Fujiwara H, Price DA, et al. Functional leukemia-associated antigen-specific memory CD8+ T cells exist in healthy individuals and in patients with chronic myelogenous leukemia before and after stem cell transplantation. Blood 2003;102:2892–900.
Rezvani K, Brenchley JM, Price DA, Kilical Y, Gostick E, Sewell AK, et al. T-cell responses directed against multiple HLA-A*0201-restricted epitopes derived from Wilms’ tumor 1 protein in patients with leukemia and healthy donors: identification, quantification, and characterization. Clin Cancer Res. 2005;11:8799–807.
Author information
Authors and Affiliations
Contributions
SL and SEH: concept, study development, and manuscript original draft. CDD, KT, JDK, HF, KF, KAL, SG, RKS, CYO, KPP, MJR, FR, LJM & SAW: Critical review of manuscript, data analysis, and resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Hussein, S., DiNardo, C.D., Takahashi, K. et al. Acquired WT1 mutations contribute to relapse of NPM1-mutated acute myeloid leukemia following allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant 57, 370–376 (2022). https://doi.org/10.1038/s41409-021-01538-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01538-w
This article is cited by
-
Mutation order in acute myeloid leukemia identifies uncommon patterns of evolution and illuminates phenotypic heterogeneity
Leukemia (2024)
-
Sex-associated differences in frequencies and prognostic impact of recurrent genetic alterations in adult acute myeloid leukemia (Alliance, AMLCG)
Leukemia (2024)