Abstract
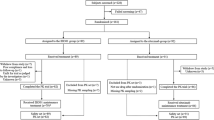
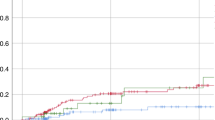
Alemtuzumab is used as part of reduced-intensity and reduced-toxicity transplant conditioning regimens for nonmalignant diseases. Prior studies identified an ideal target concentration range of 0.15–0.6 mcg/mL at day 0. However, only 24% of patients fall within this window using standard intermediate dosing. We performed a pilot study of a novel target concentration intervention strategy to target day 0 alemtuzumab concentrations to 0.15–0.6 mcg/mL. Twelve patients received model-informed alemtuzumab dosing of 0.5–0.6 mcg/kg divided over days –14 to –12. Alemtuzumab concentrations were measured, and pharmacokinetic (PK) modeling was performed on day –5 to predict day 0 concentrations. If the day 0 alemtuzumab concentration was predicted to fall below 0.15 mcg/mL, simulations were performed to identify the individual “top-up” dose needed to achieve the target day 0 concentration window. Six (50%) patients achieved day 0 alemtuzumab concentrations between 0.15 and 0.6 mcg/mL (4 received a top-up dose). Five patients had day 0 concentrations above the target window (no top-up doses). One patient had a day 0 concentration below the target range in the presence of anti-alemtuzumab antibodies. A concentration intervention strategy approach to alemtuzumab treatment can successfully target a greater proportion of patients into the ideal therapeutic window. Additional dose-reduction studies are needed to further optimize the initial dosing and achieve target attainment in all patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Chakraverty R, Orti G, Roughton M, Shen J, Fielding A, Kottaridis P. et al. Impact of in vivo alemtuzumab dose before reduced intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–8. https://doi.org/10.1182/blood-2010-05-286856.
Marsh RA, Kim MO, Liu C, Bellman D, Hart L, Grimley M. et al. An intermediate alemtuzumab schedule reduces the incidence of mixed chimerism following reduced-intensity conditioning hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transpl. 2013;19:1625–31. https://doi.org/10.1016/j.bbmt.2013.09.001.
Gartner F, Hieke S, Finke J, Bertz H. Lowering the alemtuzumab dose in reduced intensity conditioning allogeneic hematopoietic cell transplantation is associated with a favorable early intense natural killer cell recovery. Cytotherapy. 2013;15:1237–44. https://doi.org/10.1016/j.jcyt.2013.05.016.
Lane JP, Evans PT, Nademi Z, Barge D, Jackson A, Hambleton S. et al. Low-dose serotherapy improves early immune reconstitution after cord blood transplantation for primary immunodeficiencies. Biol Blood Marrow Transpl. 2014;20:243–9. https://doi.org/10.1016/j.bbmt.2013.11.005.
Marsh RA, Lane A, Mehta PA, Neumeier L, Jodele S, Davies SM. et al. Alemtuzumab levels impact acute GVHD, mixed chimerism, and lymphocyte recovery following alemtuzumab, fludarabine, and melphalan RIC HCT. Blood. 2016;127:503–12. https://doi.org/10.1182/blood-2015-07-659672.
Marsh RA, Fukuda T, Emoto C, Neumeier L, Khandelwal P, Chandra S. et al. Pretransplant Absolute Lymphocyte Counts Impact the Pharmacokinetics of Alemtuzumab. Biol Blood Marrow Transpl. 2017;23:635–41. https://doi.org/10.1016/j.bbmt.2017.01.071.
Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH. et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–6. https://doi.org/10.1182/blood-2002-09-2687.
Holford N, Ma G, Metz D. TDM is dead. Long live TCI! Br J Clin Pharmacol. 2020. https://doi.org/10.1111/bcp.14434
Dong M, Emoto C, Fukuda T, Arnold DE, Mehta PA, Marsh RA. et al. Model-informed precision dosing for alemtuzumab in pediatric and young adult patients undergoing allogeneic hematopoietic cell transplantation. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.14955
Acknowledgements
This work was supported by a Cincinnati Children’s Research Foundation GAP award.
Author information
Authors and Affiliations
Contributions
DA, CE, TF, MD, AV, PM, and RM analyzed data and wrote the manuscript. CE, TF, and MD performed modeling and simulation work. PM and AV contributed expertise and supervision of modeling and simulation work. RM and KM coordinated clinical aspects of the study. AL supervised statistical analyses for the manuscript. LN and RM measured or supervised alemtuzumab levels, respectively. ACL and FA measured anti-alemtuzumab antibodies. ATC coordinated pharmacy aspects of the study. SC, MBJ, ASN, KCM, SMD, PAM, and RAM contributed patients. All authors reviewed and contributed to editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
SMD receives research funding from Alexion and is a consultant with Novartis Pharmaceuticals. KCM is a consultant with Novartis Pharmaceuticals. The remaining authors have no relevant conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Arnold, D.E., Emoto, C., Fukuda, T. et al. A prospective pilot study of a novel alemtuzumab target concentration intervention strategy. Bone Marrow Transplant 56, 3029–3031 (2021). https://doi.org/10.1038/s41409-021-01460-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01460-1