Abstract
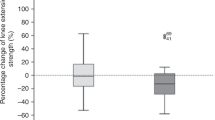
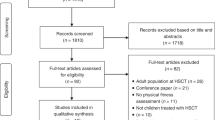
We conducted a prospective study of adult allogeneic hematopoietic cell transplantation (HCT) recipients to assess pre- and post-HCT physical function. Baseline measurements included a wrist actigraphy, a 6 min walk test (6MWT), an international physical activity questionnaire (IPAQ), and a Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) as well as serial post-HCT assessments of 6MWT, IPAQ, and FACT-BMT. Forty-seven patients were evaluable for functionality assessments, with a median follow-up of 54.5 months for surviving recipients. No patients demonstrated vigorous or very vigorous activity at any time during monitoring by wrist actigraphy; patients spent a median of 6 h daily sedentary. Self-reported activity via the IPAQ showed 36%, 43%, and 21% of subjects reporting light, moderate, and vigorous activity prior to HCT, respectively. Post-HCT 6MWTs on day +30 demonstrated the greatest association with subsequent survival and non-relapse mortality. A decline in 6MWT distance over time also demonstrated worsened overall survival. This study shows the feasibility of fitness assessments and the ability to risk stratify for subsequent mortality, particularly using the 6MWT on the day +30 single time point assessment and change scores from baseline to day +30 post HCT. These pilot findings suggest important targets for future study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Artz AS. From biology to clinical practice: aging and hematopoietic cell transplantation. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2012;18 1 Suppl:S40–5. https://doi.org/10.1016/j.bbmt.2011.11.003.
Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–52. https://doi.org/10.1182/blood-2007-07-098483.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. https://doi.org/10.1182/blood-2005-05-2004.
Sorror M, Storer B, Sandmaier BM, Maloney DG, Chauncey TR, Langston A, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer. 2008;112:1992–2001. https://doi.org/10.1002/cncr.23375.
Muffly LS, Boulukos M, Swanson K, Kocherginsky M, Cerro PD, Schroeder L, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2013;19:429–34. https://doi.org/10.1016/j.bbmt.2012.11.006.
Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol: Off J Am Soc Clin Oncol. 2013;31:876–85. https://doi.org/10.1200/JCO.2012.45.9735.
Wood WA, Le-Rademacher J, Syrjala KL, Jim H, Jacobsen PB, Knight JM, et al. Patient-reported physical functioning predicts the success of hematopoietic cell transplantation (BMT CTN 0902). Cancer. 2016;122:91–98. https://doi.org/10.1002/cncr.29717.
Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:e418–500.
Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2009;120:e169–276. https://doi.org/10.1161/CIRCULATIONAHA.109.192690.
Wood WA, Deal AM, Reeve BB, Abernethy AP, Basch E, Mitchell SA, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013;48:1342–9. https://doi.org/10.1038/bmt.2013.58.
Wingard JR, Wood WA, Martens M, Le-Rademacher J, Logan B, Knight JM, et al. Pretransplantation exercise and hematopoietic cell transplantation survival: a secondary analysis of Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0902). Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2017;23:161–4. https://doi.org/10.1016/j.bbmt.2016.10.007.
Danaher EH, Ferrans C, Verlen E, Ravandi F, van Besien K, Gelms J, et al. Fatigue and physical activity in patients undergoing hematopoietic stem cell transplant. Oncol Nurs Forum. 2006;33:614–24. https://doi.org/10.1188/06.ONF.614-624.
Bevans MF, Marden S, Leidy NK, Soeken K, Cusack G, Rivera P, et al. Health-related quality of life in patients receiving reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:101–9. https://doi.org/10.1038/sj.bmt.1705406.
Altmaier EM, Ewell M, McQuellon R, Geller N, Carter SL, Henslee-Downey J, et al. The effect of unrelated donor marrow transplantation on health-related quality of life: a report of the unrelated donor marrow transplantation trial (T-cell depletion trial). Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2006;12:648–55. https://doi.org/10.1016/j.bbmt.2006.01.003.
Andrykowski MA, Bishop MM, Hahn EA, Cella DF, Beaumont JL, Brady MJ, et al. Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. J Clin Oncol: Off J Am Soc Clin Oncol. 2005;23:599–608. https://doi.org/10.1200/JCO.2005.03.189.
Bush NE, Donaldson GW, Haberman MH, Dacanay R, Sullivan KM. Conditional and unconditional estimation of multidimensional quality of life after hematopoietic stem cell transplantation: a longitudinal follow-up of 415 patients. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2000;6:576–91.
Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers ME, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004;291:2335–43. https://doi.org/10.1001/jama.291.19.2335.
Jacobsen PB, Le-Rademacher J, Jim H, Syrjala K, Wingard JR, Logan B, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2014;20:1530–6. https://doi.org/10.1016/j.bbmt.2014.05.027.
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. https://doi.org/10.1249/01.Mss.0000078924.61453.Fb.
McQuellon RP, Russell GB, Cella DF, Craven BL, Brady M, Bonomi A, et al. Quality of life measurement in bone marrow transplantation: development of the Functional Assessment of Cancer Therapy-Bone Marrow Transplant (FACT-BMT) scale. Bone Marrow Transplant. 1997;19:357–68. https://doi.org/10.1038/sj.bmt.1700672.
Crouter SE, Kuffel E, Haas JD, Frongillo EA, Bassett DR Jr. Refined two-regression model for the ActiGraph accelerometer. Med Sci Sports Exerc. 2010;42:1029–37. https://doi.org/10.1249/MSS.0b013e3181c37458.
Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14:411–6. https://doi.org/10.1016/j.jsams.2011.04.003.
Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81.
Burr JF, Bredin SS, Faktor MD, Warburton DE. The 6-minute walk test as a predictor of objectively measured aerobic fitness in healthy working-aged adults. Phys Sportsmed. 2011;39:133–9.
Solway SBD, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–70.
Ross RM, Murthy JN, Wollak ID, Jackson AS. The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med. 2010;10:31. https://doi.org/10.1186/1471-2466-10-31.
ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. https://doi.org/10.1164/ajrccm.166.1.at1102.
Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–7. https://doi.org/10.1164/ajrccm.158.5.9710086.
Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71 2 Suppl:S114–20.
Johnson-Kozlow MSJ, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. https://doi.org/10.1182/blood-2014-01-552984.
Ainsworth BE, Haskell WL, Leon AS, Jacobs DR Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80.
Casanova C, Celli BR, Barria P, Casas A, Cote C, de Torres JP, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150–6. https://doi.org/10.1183/09031936.00194909.
Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–82. https://doi.org/10.1164/ajrccm.155.4.9105067.
Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631–6. https://doi.org/10.1055/s-0032-1323746.
Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7. https://doi.org/10.1186/1479-5868-3-7.
Cerin E, Cain KL, Oyeyemi AL, Owen N, Conway TL, Cochrane T, et al. Correlates of agreement between accelerometry and self-reported physical activity. Med Sci Sports Exerc. 2016;48:1075–84. https://doi.org/10.1249/MSS.0000000000000870.
Mishra A, Yue B, Kim J, Anasetti C, Pidala JA, Riches ML. et al. Physical activity as a predictor of outcomes in hematopoietic stem cell transplantation (HSCT) recipients. J Clin Oncol: Off J Am Soc Clin Oncol. 2015;33 15_Suppl:7027. https://doi.org/10.1200/jco.2015.33.15_suppl.7027.
Jayani R, Pidala J, Jim H, Whiting J, Mo Q, MIshra A. Association of patient-reported physical activity on allogeneic hematopoietic cell transplant outcomes. Clin Hematol Int. 2021;3:34–39.
Jones LW, Devlin SM, Maloy MA, Wood WA, Tuohy S, Espiritu N, et al. Prognostic importance of pretransplant functional capacity after allogeneic hematopoietic cell transplantation. Oncologist. 2015;20:1290–7. https://doi.org/10.1634/theoncologist.2015-0200.
Bhasin S, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriat Soc. 2020. https://doi.org/10.1111/jgs.16372.
Knols RH, de Bruin ED, Uebelhart D, Aufdemkampe G, Schanz U, Stenner-Liewen F, et al. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplant. 2011;46:1245–55. https://doi.org/10.1038/bmt.2010.288.
Hoogland AI, Bulls HW, Gonzalez BD, Small BJ, Liu L, Pidala J, et al. Circadian rhythmicity as a predictor of quality of life in allogeneic hematopoietic cell transplant patients. J Pain Symptom Manag. 2019;57:952–60.e1. https://doi.org/10.1016/j.jpainsymman.2019.01.015.
Acknowledgements
The authors wish to thank all the patients and their families as well as all physicians, nurses, medical providers, and data managers for their contribution to this study. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Bone Marrow Transplantation. This work has been supported in part by the Biostatistics and Bioinformatics Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center (P30-CA076292). Editorial assistance was provided by the Moffitt Cancer Center’s Office of Scientific Writing by Dr Paul Fletcher & Daley Drucker. No compensation was given beyond their regular salaries.
Funding
We acknowledge the following funding support: H. Lee Moffitt Cancer Center BMT-CI Foundation Grant (to AM), Moffitt Cancer Center Support Grant P30-CA076292.
Author information
Authors and Affiliations
Contributions
AM, CA, and HJ were involved with conceptualization and methodology. RT and XW contributed to the formal analysis. AM and JP contributed to the writing of the original draft and all remaining authors contributed to the draft revisions
Corresponding author
Ethics declarations
Competing interests
AM receives grant funding from Novartis. BCB is a co-inventor for provisional patents related to the use of the CD83 CAR T cells (WO2019165156), JAK inhibitors (WO2017058950A1), and STAT3 inhibitors (WO2015120436A2) in GVHD prevention and treatment; BCB nor his institution(s) have received payment related to claims described in the patents. HF is on the advisory boards for Incyte and Jazz and speakers bureau for Sanofi. FLL is a scientific advisor for Kite/Gilead, Novartis, BMS/Celgene, Allogene, Wugen, Calibr, and GammaDelta Therapeutics, is a consultant for Cellular BioMedicine Group Inc., and has institutional patents for Survivin Dendritic Cell Vaccine; improving CAR T Cell Therapy. TN receives research support to the institution (not to the individual) from Novartis and Karyopharm. HJ consulted for RedHill BioPharma, Janssen Scientific Affairs, and Merck. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mishra, A., Pidala, J., Thapa, R. et al. Objective and subjective physical function in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant 56, 2897–2903 (2021). https://doi.org/10.1038/s41409-021-01428-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01428-1
This article is cited by
-
Rehabilitation Implications of Cellular Therapy for Cancer
Current Physical Medicine and Rehabilitation Reports (2024)
-
A predictive model combining clinical characteristics and nutritional risk factors for overall survival after umbilical cord blood transplantation
Stem Cell Research & Therapy (2023)
-
The immediate impact of physical function and quality of life after hematopoietic stem cell transplantation
Supportive Care in Cancer (2022)
-
Rehabilitation Needs for Patients Undergoing CAR T-Cell Therapy
Current Oncology Reports (2022)
-
Physically “fit” for allogeneic stem cell transplant?
Bone Marrow Transplantation (2021)