Abstract
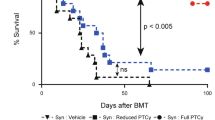
We assessed the impact of the Janus Kinase (JAK) 1 inhibitor itacitinib on xenogeneic graft-versus-host disease (xGVHD). XGVHD was induced by i.v. injection 20 × 106 human peripheral blood mononuclear cells (hPBMC) in NSG mice on day 0. Itacitinib (3 mg, ≈120 mg/kg) or methylcellulose was administered by force-feeding twice a day from day 3 to day 28. Mice were followed for xGVHD score and survival. In addition, human T-cell engraftment and as well as human T-cell subtypes were monitored in blood on days 14, 21, and 28 after transplantation. We observed that itacitinib-treated mice had significantly longer survival than control mice (median 45 versus 33 days; P < 0.001). Further, they also had lower absolute numbers of human CD4+ T cells on days 21 and 28 after transplantation as well as of human CD8+ T cells on days 14, 21, and 28 after transplantation. In addition, itacitinib-treated mice had higher frequencies of human regulatory T cells (Treg) on days 21 and 28 after transplantation. In summary, our data indicate that itacitinib decreases human T-cell engraftment, increases Treg frequencies and attenuates xGVHD in NSG mice transplanted with hPBMC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets supporting the conclusions of this article are included within the article and its additional files. Raw files used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baron F, Efficace F, Cannella L, Muus P, Trisolini S, Halkes CJM, et al. Impact of the type of anthracycline and of stem cell transplantation in younger patients with acute myeloid leukemia: Long-term follow up of a phase III study. Am J Hematol. 2020;95:749–58.
Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003.
Hammarén HM, Virtanen AT, Raivola J, Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine. 2019;118:48–63.
Schroeder MA, Choi J, Staser K, DiPersio JF. The role of janus kinase signaling in graft-versus-host disease and graft versus leukemia. Biol Blood Marrow Transpl. 2018;24:1125–34.
Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl J Med. 2020;382:1800–10.
Covington M, He X, Scuron M, Li J, Collins R, Juvekar A, et al. Preclinical characterization of itacitinib (INCB039110), a novel selective inhibitor of JAK1, for the treatment of inflammatory diseases. Eur J Pharm. 2020;885:173505.
Schroeder MA, Khoury HJ, Jagasia M, Ali H, Schiller GJ, Staser K, et al. A phase 1 trial of itacitinib, a selective JAK1 inhibitor, in patients with acute graft-versus-host disease. Blood Adv. 2020;4:1656–69.
Zeiser R, Socié G, Schroeder MA, Abhyankar S, Pinho Vaz C, Kwon M et al. GRAVITAS-301: A Randomized, double-blind phase 3 study of itacitinib or placebo in combination with corticosteroids for initial treatment of patients with acute graft-versus-host disease. EHA Library 2020;: S256.
King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–18.
Hannon M, Lechanteur C, Lucas S, Somja J, Seidel L, Belle L, et al. Infusion of clinical-grade enriched regulatory T cells delays experimental xenogeneic graft-versus-host disease. Transfusion. 2014;54:353–63.
Gregoire C, Ritacco C, Hannon M, Seidel L, Delens L, Belle L, et al. Comparison of mesenchymal stromal cells from different origins for the treatment of graft-vs.-host-disease in a humanized mouse model. Front Immunol. 2019;10:619.
Ehx G, Somja J, Warnatz H-J, Ritacco C, Hannon M, Delens L, et al. Xenogeneic graft-versus-host disease in humanized NSG and NSG-HLA-A2/HHD mice. Front Immunol. 2018;9:1943.
Ehx G, Fransolet G, de Leval L, D’Hondt S, Lucas S, Hannon M, et al. Azacytidine prevents experimental xenogeneic graft-versus-host disease without abrogating graft-versus-leukemia effects. Oncoimmunology. 2017;6:e1314425.
Pierson W, Cauwe B, Policheni A, Schlenner SM, Franckaert D, Berges J, et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:959–65.
Kawasaki Y, Sato K, Hayakawa H, Takayama N, Nakano H, Ito R, et al. Comprehensive analysis of the activation and proliferation kinetics and effector functions of human lymphocytes, and antigen presentation capacity of antigen-presenting cells in xenogeneic graft-versus-host disease. Biol Blood Marrow Transpl. 2018;24:1563–74.
Brehm MA, Kenney LL, Wiles MV, Low BE, Tisch RM, Burzenski L, et al. Lack of acute xenogeneic graft- versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 2019;33:3137–51.
Coman T, Rossignol J, D’Aveni M, Fabiani B, Dussiot M, Rignault R, et al. Human CD4- invariant NKT lymphocytes regulate graft versus host disease. Oncoimmunology. 2018;7:e1470735.
Søndergaard H, Kvist PH, Haase C. Human T cells depend on functional calcineurin, tumour necrosis factor-α and CD80/CD86 for expansion and activation in mice. Clin Exp Immunol. 2013;172:300–10.
Pérol L, Martin GH, Maury S, Cohen JL, Piaggio E. Potential limitations of IL-2 administration for the treatment of experimental acute graft-versus-host disease. Immunol Lett. 2014;162:173–84.
Abraham S, Guo H, Choi J-G, Ye C, Thomas MB, Ortega N, et al. Combination of IL-10 and IL-2 induces oligoclonal human CD4 T cell expansion during xenogeneic and allogeneic GVHD in humanized mice. Heliyon. 2017;3:e00276.
Delens L, Ehx G, Somja J, Vrancken L, Belle L, Seidel L, et al. In Vitro Th17-Polarized Human CD4(+) T Cells Exacerbate Xenogeneic Graft-versus-Host Disease. Biol Blood Marrow Transpl. 2019;25:204–15.
Ehx G, Ritacco C, Hannon M, Dubois S, Delens L, Willems E et al. Comprehensive analysis of the immunomodulatory effects of rapamycin on human T cells in graft-versus-host disease prophylaxis. Am J Transplant. 2021. https://doi.org/10.1111/ajt.16505.
Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–42.
Betts BC, Veerapathran A, Pidala J, Yang H, Horna P, Walton K et al. Targeting Aurora kinase A and JAK2 prevents GVHD while maintaining Treg and antitumor CTL function. Sci Transl Med 2017; 9. https://doi.org/10.1126/scitranslmed.aai8269.
Wang H, Song H, Pham AV, Cooper LJ, Schulze JJ, Olek S, et al. Human LAP(+)GARP(+)FOXP3(+) regulatory T cells attenuate xenogeneic graft versus host disease. Theranostics. 2019;9:2315–24.
Cuende J, Liénart S, Dedobbeleer O, van der Woning B, De Boeck G, Stockis J, et al. Monoclonal antibodies against GARP/TGF-β1 complexes inhibit the immunosuppressive activity of human regulatory T cells in vivo. Sci Transl Med. 2015;7:284ra56.
Liston A, Gray DHD. Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 2014;14:154–65.
Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–9.
Zeiser R, Negrin RS. Interleukin-2 receptor downstream events in regulatory T cells: implications for the choice of immunosuppressive drug therapy. Cell Cycle. 2008;7:458–62.
Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62.
Ringdén O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–8.
Baron F, Labopin M, Savani BN, Beohou E, Niederwieser D, Eder M, et al. Graft-versus-host disease and graft-versus-leukaemia effects in secondary acute myeloid leukaemia: a retrospective, multicentre registry analysis from the Acute Leukaemia Working Party of the EBMT. Br J Haematol. 2020;188:428–37.
Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transpl. 2011;11:1148–57.
Boucault L, Lopez Robles M-D, Thiolat A, Bézie S, Schmueck-Henneresse M, Braudeau C, et al. Transient antibody targeting of CD45RC inhibits the development of graft-versus-host disease. Blood Adv. 2020;4:2501–15.
Acknowledgements
We are grateful to Sandra Ormenese, Raafat Stephan, and Céline Vanwinge from the Imaging and Flow Cytometry Platform of the GIGA for help with flow cytometry analyses. We are also grateful to INCYTE Biosciences who kindly gave us itacitinib.
Funding
This study was supported by funds from: the National Fund for Scientific Research (FNRS) (grant numbers T.0069.15 and T.0016.20), The Belgian Fondation contre le cancer (grant # FBC # FAF-C/2016/889), and the Leon Fredericq fund and Anti-Cancer Center at the University of Liège. GE is a Télévie Research Assistant, and FB is a senior research associate of the National Fund for Scientific Research (FNRS) Belgium.
Author information
Authors and Affiliations
Contributions
FB designed the study; JC, CR, and SD performed the experiments; LC, BV, and CD helped in the experiments; JC, LS and CR analyzed the data; JC, CR, GE, and FB interpreted the data; SS, JCA and YB helped in data interpretation; FB and JC wrote the article; all authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Frédéric Baron has received travel grants from Celgene, Abbvie, Novartis, INCYTE Biosciences, and Sanofi as well as honoraria from Merck and Abbvie. The remaining authors declare that they have no relevant conflict of interest in regard to this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Courtois, J., Ritacco, C., Dubois, S. et al. Itacitinib prevents xenogeneic GVHD in humanized mice. Bone Marrow Transplant 56, 2672–2681 (2021). https://doi.org/10.1038/s41409-021-01363-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01363-1
This article is cited by
-
Patient-derived tumor models: a suitable tool for preclinical studies on esophageal cancer
Cancer Gene Therapy (2023)
-
PD-1-CD28 fusion protein strengthens mesothelin-specific TRuC T cells in preclinical solid tumor models
Cellular Oncology (2023)