Abstract
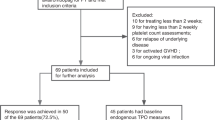
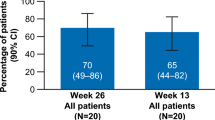
Eltrombopag has shown efficacy in the treatment of thrombocytopenia and poor graft function (PGF) after allogeneic hematopoietic cell transplantation (HCT) in retrospective observational studies, but is not approved for this indication. The cost of this drug is also a major concern in publicly funded health care systems. We collected data about patients who received eltrombopag for thrombocytopenia or PGF after HCT. Post-HCT thrombocytopenia, PGF, and eltrombopag response were defined as per previously published criteria. Primary outcome was treatment efficacy and secondary outcome was cost comparison between estimated treatment cost prior to and after initiation of eltrombopag. Seventeen patients (males 70.6%; median age = 58) received eltrombopag. Isolated thrombocytopenia was present in 11.8% (n = 2) patients while PGF was present in 88.2% (n = 15) of patients. After 8 weeks of treatment at the maximum dose of 150 mg orally daily, overall response rate (ORR) was seen in 76.5% (13/17) of patients: complete response (CR) in 10/13 patients and partial response (PR) in 3/13 patients. The use of eltrombopag was associated with an overall decrease in the total weekly care costs (5021 vs 2,524 CA$; P = 0.04). Thus, Eltrombopag is an efficacious and possibly cost-effective therapy for thrombocytopenia and PGF after allogeneic HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nash RA, Gooley T, Davis C, Appelbaum FR. The problem of thrombocytopenia after hematopoietic stem cell transplantation. Stem Cells. 1996;14:261–73.
Akahoshi Y, Kanda J, Gomyo A, Hayakawa J, Komiya Y, Harada N, et al. Risk factors and impact of secondary failure of platelet recovery after. Biol Blood Marrow Transplant. 2016;22:1678–83.
Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020;26:e65–e73.
Bruno B, Gooley T, Sullivan KM, Davis C, Bensinger WI, Storb R, et al. Secondary failure of platelet recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:154–62.
Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation-frequency and outcomes. Bone Marrow Transplant. 2004;33:729–34.
Davies SM, Kollman C, Anasetti C, Antin JH, Gajewski J, Casper JT, et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the national marrow donor program. Blood. 2000;96:4096–102.
Dominietto A, Raiola AM, van Lint MT, Lamparelli T, Gualandi F, Berisso G, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001;112:219–27.
Bolwell B, Pohlman B, Sobecks R, Andresen S, Brown S, Rybicki L, et al. Prognostic importance of the platelet count 100 days post allogeneic bone marrow transplant. Bone Marrow Transplant. 2004;33:419–23.
First LR, Smith BR, Lipton J, Nathan DG, Parkman R, Rappeport JM. Isolated thrombocytopenia after allogeneic bone marrow transplantation: existence of transient and chronic thrombocytopenic syndromes. Blood. 1985;65:368–74.
Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1440–3.
Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. 2007;109:4739–41.
Imbach P, Crowther M. Thrombopoietin-receptor agonists for primary immune thrombocytopenia. N Engl J Med. 2011;365:734–41.
Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357:2237–47.
McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–36.
Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11–9.
Rivera D, Bastida JM, Lopez-Corral L, Sanchez-Guijo F, Cabrero M, Martin A, et al. Usefulness of eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2019;54:757–61.
Samarkandi H, Al Nahedh M, Alfattani A, Alsharif F, Bakshi N, Rasheed W, et al. Evaluation of eltrombopag in thrombocytopenia post Hematopoietic cell transplantation: rertrospective observational trial. Hematol Oncol Stem Cell Ther. 2020;30:S1658–3876. https://doi.org/10.1016/j.hemonc.2020.07.006. 30123-0.
Halahleh K, Gale RP, Da’na W, Ma’koseh M, Saadeh S, Alan W, et al. Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplant. 2021;56:4–6.
Fu H, Zhang X, Han T, Mo X, Wang Y, Chen H, et al. Eltrombopag is an effective and safe therapy for refractory thrombocytopenia after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54:1310–8.
Aydin S, Dellacasa C, Manetta S, Giaccone L, Godio L, Iovino G, et al. Rescue treatment with eltrombopag in refractory cytopenias after allogeneic stem cell transplantation. Ther Adv Hematol 2020;11:2040620720961910. https://doi.org/10.1177/2040620720961910.
Blood Component Cost Per Unit Summary—Annual Report. Canadian Blood Services. 2018–2019. https://annual2019.blood.ca/pdfs/CBS-AR2019-en.pdf. Accessed 14 Apr 2021.
Zhang X, Fu H, Xu L, Liu D, Wang J, Liu K, et al. Prolonged thrombocytopenia following allogeneic hematopoietic stem cell transplantation and its association with a reduction in ploidy and an immaturation of megakaryocytes. Biol Blood Marrow Transplant. 2011;17:274–80.
Yamazaki R, Kuwana M, Mori T, Okazaki Y, Kawakami Y, Ikeda Y, et al. Prolonged thrombocytopenia after allogeneic hematopoietic stem cell transplantation: associations with impaired platelet production and increased platelet turnover. Bone Marrow Transplant. 2006;38:377–84.
Koike Y, Yoneyama A, Shirai J, Ishida T, Shoda E, Miyazaki K, et al. Evaluation of thrombopoiesis in thrombocytopenic disorders by simultaneous measurement of reticulated platelets of whole blood and serum thrombopoietin concentrations. Thrombosis Haemost. 1998;79:1106–10.
Vasudevan Nampoothiri R, Kumar R. Eltrombopag: Role in cytopenias following hematopoietic stem cell transplantation. Indian J Hematol Blood Transfus. 2020;36:238–45.
Will B, Kawahara M, Luciano JP, Bruns I, Parekh S, Erickson-Miller CL, et al. Effect of the nonpeptide thrombopoietin receptor agonist Eltrombopag on bone marrow cells from patients with acute myeloid leukemia and myelodysplastic syndrome. Blood. 2009;114:3899.
Vlachodimitropoulou E, Chen YL, Garbowski M, Koonyosying P, Psaila B, Sola-Visner M, et al. Eltrombopag: a powerful chelator of cellular or extracellular iron(III) alone or combined with a second chelator. Blood. 2017;130:1923–33.
Alvarado LJ, Huntsman HD, Cheng H, Townsley DM, Winkler T, Feng X, et al. Eltrombopag maintains human hematopoietic stem and progenitor cells under inflammatory conditions mediated by IFN-γ. Blood. 2019;133:2043–55.
Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116:4639–45.
Schifferli A, Kühne T. Thrombopoietin receptor agonists: a new immune modulatory strategy in immune thrombocytopenia? Semin Hematol. 2016;53:S31–4.
Reid R, Bennett JM, Becker M, Chen Y, Milner L, Phillips GL 2nd, et al. Use of eltrombopag, a thrombopoietin receptor agonist, in post-transplantation thrombocytopenia. Am J Hematol. 2012;87:743–5.
Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22:919–24.
Dyba J, Tinmouth A, Bredeson C, Matthews J, Allan DS. Eltrombopag after allogeneic haematopoietic cell transplantation in a case of poor graft function and systematic review of the literature. Transfus Med. 2016;26:202–7.
Tang C, Chen F, Kong D, Ma Q, Dai H, Yin J, et al. Successful treatment of secondary poor graft function post allogeneic hematopoietic stem cell transplantation with eltrombopag. J Hematol Oncol. 2018;11:103.
Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123:1818–25.
Bento L, Bastida JM, García-Cadenas I, García-Torres E, Rivera D, Bosch-Vilaseca A, et al. Thrombopoietin receptor agonists for severe thrombocytopenia after allogeneic stem cell transplantation: experience of the Spanish Group of Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019;25:1825–31.
Yuan C, Boyd AM, Nelson J, Patel RD, Varela JC, Goldstein SC, et al. Eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1320–4.
Ghanima W, Geyer JT, Lee CS, Boiocchi L, Imahiyerobo AA, Orazi A, et al. Bone marrow fibrosis in 66 patients with immune thrombocytopenia treated with thrombopoietin-receptor agonists: a single-center, long-term follow-up. Haematologica. 2014;99:937–44.
Smieliauskas F, Chien CR, Shen C, Geynisman DM, Shih YC. Cost-effectiveness analyses of targeted oral anti-cancer drugs: a systematic review. PharmacoEconomics. 2014;32:651–80.
Raut SS, Shah SA, Sharanangat VV, Shah KM, Patel KA, Anand AS, et al. Safety and efficacy of eltrombopag in post-hematopoietic stem cell transplantation (HSCT) thrombocytopenia. Indian J Hematol Blood Transfus. 2015;31:413–5.
Funding
There is no funding source to disclose for this study. Few of the patients received compassionate use of the drug Eltrombopag from NovartisTM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nampoothiri, R.V., Ho, L., McEwan, C. et al. Efficacy and cost analysis of eltrombopag in thrombocytopenia and poor graft function post allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 56, 2471–2476 (2021). https://doi.org/10.1038/s41409-021-01362-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-021-01362-2
This article is cited by
-
Eltrombopag may be cost effective for thrombocytopenia and PGF post transplantation
PharmacoEconomics & Outcomes News (2021)