Abstract
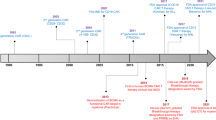
In the United States the increasing number of Food and Drug Administration (FDA)-approved, innovative, and potentially effective commercial cancer therapies pose a significant financial burden on public and private payers. Chimeric antigen receptor (CAR) T cells are prototypical of this challenge. In 2017 and 2018, tisagenlecleucel (Kymriah, Novartis) and axicabtagene ciloleucel (Yescarta, Kite) were approved by the FDA for use after showing groundbreaking results in relapsed/refractory B-cell malignancies. In 2020 and 2021, four further submissions to the FDA are expected for CAR T-cell therapies for indolent and aggressive B-cell malignancies and plasma cell myeloma. Yet, with marketed prices of over $350,000 per infusion for the two FDA-approved therapies and similar price tags expected for the coming products, serious concerns are raised over value and affordability. In this review we summarize recent, peer-reviewed cost-effectiveness studies of tisagenlecleucel and axicabtagene ciloleucel in the United States; discuss key issues concerning the health plan budget impact of CAR T-cell therapy; and review policy, payment and scientific approaches that may improve the value and affordability of CAR T-cell therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–81.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
Health Canada. Register of innovative drugs. Government of Canada, Ottawa, Canada; 2019.
Therapeutic Goods Administration. 312686: Cellular Therapies - T Cells - Tisagenlecleucel, cryopreserved - T - Kymriah - Novartis Pharmaceuticals Australia Pty Ltd - Suspension - Bag. Canberra, Australian Capital Territory: Department of Health; 2018.
Therapeutic Goods Administration. 312685: Cellular Therapies - T Cells - Tisagenlecleucel, cryopreserved - T - Kymriah - Novartis Pharmaceuticals Australia Pty Ltd - Suspension - Bag. Canberra, Australian Capital Territory: Department of Health; 2018.
European Medicines Agency. Press Release: first two CAR-T cell medicines recommended for approval in the European Union. Amsterdam: European Medicines Agency; 2018.
National Health Service. CAR-T therapy. National Health Service, Redditch, England; 2019.
Hernandez I, Prasad V, Gellad WF. Total costs of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4:994–6.
Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36:3192–202.
Roth JA, Sullivan SD, Lin VW, Bansal A, Purdum AG, Navale L, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018;21:1238–45.
Sarkar RR, Gloude NJ, Schiff D, Murphy JD. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. J Natl Cancer Inst. 2018;111:719–26.
Whittington MD, McQueen RB, Ollendorf DA, Kumar VM, Chapman RH, Tice JA, et al. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018;172:1161–8.
Whittington MD, McQueen RB, Ollendorf DA, Kumar VM, Chapman RH, Tice JA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019;2:e190035.
Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber-Fiebert JD. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019;37:2105–19.
Dearment A. Celgene filing plans potentially mean two more CAR-Ts on the market by end of next year. MedCity News; 2019. Accessed at: https://medcitynews.com/2019/07/celgene-filing-plans-potentially-mean-two-more-car-ts-on-the-market-by-end-of-next-year. Last accessed on: May 27, 2020.
Tucker N. BLA submitted to FDA for KTE-X19 as treatment of relapsed/refractory MCL. Targeted Oncology; 2019. Accessed at: https://www.targetedonc.com/view/bla-submitted-to-fda-for-ktex19-as-treatment-of-relapsedrefractory-mcl. Last accessed on: May 27, 2020.
Maude SL, Pulsipher MA, Boyer MW, Grupp SA, Davies SM, Phillips CL, et al. Efficacy and safety of CTL019 in the first US Phase II Multicenter Trial in pediatric relapsed/refractory acute lymphoblastic leukemia: results of an interim analysis. Blood. 2016;128:2801.
Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47.
Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8.
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang ML, Arnason JE, et al. Pivotal safety and efficacy results from transcend NHL 001, a Multicenter Phase 1 Study of lisocabtagene maraleucel (liso-cel) in relapsed/refractory (R/R) large B cell lymphomas In: American Society of Hematology Annual Meeting. Orlando, FL.: American Society of Hematology; 2019.
Siddiqi T, Soumerai J, Dorritie KA, Stephens DM, Riedell PA, Arnason JE, et al. Rapid undetectable MRD (uMRD) responses in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) treated with lisocabtagene maraleucel (liso-cel), a CD19-directed CAR T cell product: updated results from transcend CLL 004, a phase 1/2 study including patients with high-risk disease previously treated with ibrutinib. American Society of Hematology Annual Meeting. Orlando, FL: American Society of Hematology; 2019.
Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331–42.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37.
Schulthess D, Gassull D, Makady A, Ludlow A, Rothman B, Have PT, et al. Are CAR-T therapies living up to their hype? A study using real-world data in two cohorts to determine how well they are actually working in practice compared with bone marrow transplants [published online ahead of print, 2019 Jul 17]. BMJ Evid Based Med. 2019;bmjebm-2019-111226. https://doi.org/10.1136/bmjebm-2019-111226.
Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–81.
Waters TM, Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Newman LA, et al. Beyond efficacy and effectiveness: conducting economic analyses during clinical trials. Dysphagia. 2004;19:109–19.
Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21.
Prieto L, Sacristán JA. Problems and solutions in calculating quality-adjusted life years (QALYs). Health Qual Life Outcomes. 2003;1:80–80.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine: recommendations from the second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Santos HGD, Zampieri FG, Normilio-Silva K, Silva GTD, Lima ACP, Cavalcanti AB, et al. Machine learning to predict 30-day quality-adjusted survival in critically ill patients with cancer. J Crit Care. 2020;55:73–78.
Gafni A, Birch S. Incremental cost-effectiveness ratios (ICERs): the silence of the lambda. Soc Sci Med. 2006;62:2091–2100.
Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Mak. 2000;20:332–42.
Nadler ES, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? J Clin Oncol. 2005;23 16_suppl:6011–6011.
Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94:925–30.
Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7.
House Office of the Legislative Counsel. Compilation of patient protection and affordable care act: extracted sections concerning patient-centered outcomes research and the authorization of the Patient-Centered Outcomes Research Institute (PCORI). In: Subtitle D of Title VI - Sec. 6301. Washington, D.C.: House Office of the Legislative Counsel; 2010.
Bryan WW. Biologics license application approval for tisagenlecleucel for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse (BL 125646/0). In: Department of Health and Human Services. Silver Spring, MD: United States Food and Drug Administration; 2017.
Malarkey MA, Bryan WW. Biologics license application approval for axicabtagene ciloleucel for the treatment of adult patients with relapsed or refractory large B cell lymphoma (BL 125643/0). In: Department of Health and Human Services. Silver Spring, MD: United States Food and Drug Administration; 2017.
Purohit-Sheth T. Biologics license application supplement approval for tisagenlecleucel for the treatment of adult patients with relapsed or refractory large B cell lymphoma after two or more lines of systemic therapy (BL 125646/76). In: Department of Health and Human Services. Silver Spring, MD: United States Food and Drug Administration; 2018.
Bansal A, Sullivan SD, Lin VW, Purdum AG, Navale L, Cheng P, et al. Estimating long-term survival for patients with relapsed or refractory large B-cell lymphoma treated with chimeric antigen receptor therapy: a comparison of standard and mixture cure models. Med Decis Mak. 2019;39:294–8.
Delea TE, Zhang X, Amdahl J, Boyko D, Dirnberger F, Campioni M, et al. Cost effectiveness of blinatumomab versus inotuzumab ozogamicin in adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia in the United States. Pharmacoeconomics. 2019;37:1177–93.
Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR-T cells. Blood. 2019;133:1652–63. https://doi.org/10.1182/blood-2018-11-883710.
Schuster SJ, Bartlett NL, Assouline S, Yoon S, Bosch F, Sehn LH, et al. Mosunetuzumab induces complete remissions in poor prognosis non-hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. In: American Society of Hematology Annual Meeting. Orlando, FL: American Society of Hematology; 2019.
Thornton Snider J, Brauer M, Kee R, Batt K, Karaca-Mandic P, Zhang J, et al. The potential impact of CAR T-cell treatment delays on society. Am J Manag Care. 2019;25:379–86.
Hargreaves B. Cell therapy market to triple in size by 2025. Crawley: William Reed Business Media Ltd; 2019.
Jürgens B, Clarke NS. Evolution of CAR T-cell immunotherapy in terms of patenting activity. Nat Biotechnol. 2019;37:370–5.
Jensen TS, Chin J, Ashby LM, Hakim R, Paserhia LA, Szarama KB. Decision memo for chimeric antigen receptor (CAR) T-cell therapy for cancers (CAG-00451N) In: Services CfMaM. Washington D.C.: cms.gov; 2019.
American Society of Hematology, American Society of Blood and Marrow Transplantation. Re: follow-up to August 30, 2018 meeting; proposed CAR-T coverage and payment options. Washington D.C.: ASH and ABMT; 2019.
Lomas J, Claxton K, Martin S, Soares M. Resolving the “cost-effective but unaffordable” paradox: estimating the health opportunity costs of nonmarginal budget impacts. Value Health. 2018;21:266–75.
Trueman P, Drummond M, Hutton J. Developing guidance for budget impact analysis. Pharmacoeconomics. 2001;19:609–21.
Hollmann S, Painter C, Hogan A, Morten P, Goyert N, Vieira J, et al. PCN72 - Budget Impact Analysis of Tisagenlecleucel for the Treatment of Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma in England. Value Health. 2018;21:S26.
Hastert TA, Young GS, Pennell ML, Padamsee T, Zafar SY, DeGraffinreid C, et al. Financial burden among older, long-term cancer survivors: Results from the LILAC study. Cancer Med. 2018;7:4261–72.
Nathan PC, Henderson TO, Kirchhoff AC, Park ER, Yabroff KR. Financial hardship and the economic effect of childhood cancer survivorship. J Clin Oncol. 2018;36:2198–205.
ASCO Post Staff. Treatment centers authorized to administer CAR T-cell therapy. In: The ASCO Post. Virginia: American Society of Clinical Oncology; 2020.
Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68:153–65.
Ho LD, Oso SO, Levine AD. Medical crowdfunding to access CAR T-cell therapy. Lancet Oncol. 2019;20:1062–4.
Sarnak DO, Squires D, Bishop S. Paying for prescription drugs around the world: why is the U.S. an outlier? New York, NY: The Commonwealth Fund; 2017.
Kang SY, Bai G, DiStefano MJ, Socal MP, Yehia F, Anderson GF. Comparative Approaches to Drug Pricing. Annu Rev Public Health. 2020;41:499–512. https://doi.org/10.1146/annurev-publhealth-040119-094305.
Parker-Lue S, Santoro M, Koski G. The ethics and economics of pharmaceutical pricing. Annu Rev Pharm Toxicol. 2015;55:191–206.
Sarpatwari A, DiBello J, Zakarian M, Najafzadeh M, Kesselheim AS. Competition and price among brand-name drugs in the same class: a systematic review of the evidence. PLoS Med. 2019;16:e1002872.
West J. Policy roundtables: competition, patents and innovation organisation for economic co-operation and development: Paris, Organisation for Economic Co-operation and Development; 2006. Accessed at: https://www.oecd.org/competition/abuse/39888509.pdf. Last accessed on: May 27, 2020.
Chen BK, Yang YT, Bennett CL. Why biologics and biosimilars remain so expensive: despite two wins for biosimilars, the Supreme Court’s recent rulings do not solve fundamental barriers to competition. Drugs. 2018;78:1777–81.
Fu M, Tang L. Chimeric antigen receptor T cell immunotherapy for tumor: a review of patent literatures. Recent Pat Anticancer Drug Discov. 2019;14:60–69.
Welch AR. New medicare proposals could impact biosimilar competition. In: Biosimilar Development. Erie, PA: Biosimilar Development; 2017.
Bach PB. National coverage analysis of CAR-T therapies - policy, evidence, and payment. N Engl J Med. 2018;379:1396–8.
Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101.
Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58:928–36.
Castella M, Boronat A, Martin-Ibanez R, Rodriguez V, Sune G, Caballero M, et al. Development of a novel anti-CD19 chimeric antigen receptor: a paradigm for an affordable CAR T cell production at academic institutions. Mol Ther Methods Clin Dev. 2019;12:134–44.
Zhang W, Jordan KR, Schulte B, Purev E. Characterization of clinical grade CD19 chimeric antigen receptor T cells produced using automated CliniMACS Prodigy system. Drug Des Devel Ther. 2018;12:3343–56.
Ramanayake S, Bilmon I, Bishop D, Dubosq MC, Blyth E, Clancy L, et al. Low-cost generation of good manufacturing practice-grade CD19-specific chimeric antigen receptor-expressing T cells using piggyBac gene transfer and patient-derived materials. Cytotherapy. 2015;17:1251–67.
Bishop DC, Xu N, Tse B, O’Brien TA, Gottlieb DJ, Dolnikov A, et al. PiggyBac-engineered T cells expressing CD19-specific CARs that lack IgG1 Fc spacers have potent activity against B-ALL xenografts. Mol Ther. 2018;26:1883–95.
Monjezi R, Miskey C, Gogishvili T, Schleef M, Schmeer M, Einsele H, et al. Enhanced CAR T-cell engineering using non-viral sleeping beauty transposition from minicircle vectors. Leukemia. 2017;31:186–94.
Skrdlant LM, Armstrong RJ, Keidaisch BM, Lorente MF, DiGiusto DL. Detection of replication competent lentivirus using a qPCR assay for VSV-G. Mol Ther Methods Clin Dev. 2018;8:1–7.
Son KB, Kim CY, Lee TJ. Understanding of for whom, under what conditions and how the compulsory licensing of pharmaceuticals works in Brazil and Thailand: a realist synthesis. Glob Public Health. 2019;14:122–34.
Dana KN, Hertig JB, Weber RJ. Drug pricing transparency: the new retail revolution. Hosp Pharm. 2017;52:155–9.
Lyman GH, Nguyen A, Snyder S, Gitlin M, Chung KC. Economic evaluation of chimeric antigen receptor T-cell therapy by site of care among patients with relapsed or refractory large B-cell lymphoma. JAMA Netw Open. 2020;3:e202072.
Cubanski J, Neuman T, True S, Freed M. What’s the latest on medicare drug price negotiations? KFF; 2019. Accessed at: https://www.kff.org/medicare/issue-brief/whats-the-latest-on-medicare-drug-price-negotiations. Last accessed on: May 27, 2020.
Sklar T, Robertson C. Affordability boards — the states’ new fix for drug pricing. N Engl J Med. 2019;381:1301–3.
Dusetzina SB, Oberlander J. Advancing legislation on drug pricing — is there a path forward? N Engl J Med. 2019;381:2081–4.
Aviki EM, Schleicher SM, Mullangi S, Matsoukas K, Korenstein D. Alternative payment and care-delivery models in oncology: a systematic review. Cancer. 2018;124:3293–306.
Ems D, Murty S, Loy B, Gallagher J, Happe LE, Rogstad TL, et al. Alternative payment models in medical oncology: assessing quality-of-care outcomes under partial capitation. Am Health Drug Benefits. 2018;11:371–8.
Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10:322–6.
Basu A. Financing cures in the United States. Expert Rev Pharmacoecon Outcomes Res. 2015;15:1–4.
Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2019;17:147–67.
Gonzalez-Fernandez M, Villamanan E, Jimenez-Nacher I, Moreno F, Plasencia C, Gaya F, et al. Cost evolution of biological agents for the treatment of spondyloarthritis in a tertiary hospital: influential factors in price. Int J Clin Pharm. 2018;40:1528–38.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASBMT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38.
Ruark J, Mullane E, Cleary N, Cordeiro A, Bezerra ED, Wu V, et al. Patient-reported neuropsychiatric outcomes of long-term survivors after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2020;26:34–43.
Whittington MD, Ollendorf DA, Campbell JD. Accounting for all costs in the total cost of chimeric antigen receptor T-cell immunotherapy. JAMA Oncol. 2018;4:1784–5.
Shalabi H, Shah NN, Fry TJ, Yates B, Delbrook C. Chimeric antigen receptor induced cytopenia differs from chemotherapy induced myelosuppression. Blood. 2017;130 Suppl 1:5048.
Ying Z, Huang XF, Xiang X, Liu Y, Kang X, Song Y, et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25:947–53.
Acknowledgements
We thank Dr Stefanie Kalfas and Dr Jordan Gauthier for valuable discussions, critical reading, and editing of the manuscript. We thank Dr Lindsie Goss for consultations regarding CAR T-cell patenting.
Funding
This study was supported in part by funds from the Fred Hutchinson Cancer Research Center Integrated Immunotherapy Research Center (PI: Roth JA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SF and DSR declare no conflicts of interest. CJT is an inventor on patents licensed or pending to Juno Therapeutics (a Bristol-Myers Squibb company) and Nektar Therapeutics; receives research funding from Juno Therapeutics (a Bristol-Myers Squibb company) and Nektar Therapeutics; has equity options in Precision Biosciences, Caribou Biosciences, and Eureka Therapeutics; and has served as an advisor for Juno Therapeutics (a Bristol-Myers Squibb company), Nektar Therapeutics, Precision Biosciences, Caribou Biosciences, Eureka Therapeutics, Allogene, Kite Pharma (a Gilead company), Novartis, Humanigen, Aptevo, and Allogene. JAR and SDR have served as consultants for Kite Pharma (a Gilead company).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fiorenza, S., Ritchie, D.S., Ramsey, S.D. et al. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transplant 55, 1706–1715 (2020). https://doi.org/10.1038/s41409-020-0956-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0956-8
This article is cited by
-
Issues, Challenges and Opportunities for Economic Evaluations of Orphan Drugs in Rare Diseases: An Umbrella Review
PharmacoEconomics (2024)
-
Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies
Journal of Hematology & Oncology (2023)
-
Remote control of cellular immunotherapy
Nature Reviews Bioengineering (2023)
-
The Gibco™ CTS™ Rotea™ system story—a case study of industry-academia collaboration
Gene Therapy (2023)
-
Two for one: targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells
Journal of Translational Medicine (2022)