Abstract
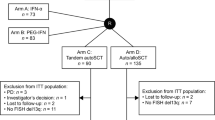
Contrary to tandem autologous transplant (auto-auto), autologous followed by reduced intensity conditioning allogenic transplantation (auto-allo) offers graft-versus-myeloma (GVM) effect but with higher toxicity. Trials comparing these two strategies relied on availability of HLA-matched sibling donors for arm allocation (biological randomization) and yielded conflicting results. A pooled analysis of multiple trials with extended follow up provides an opportunity to compare these strategies. We obtained individual patient data from participants of four trials comparing auto-auto vs. auto-allo after induction therapy. There were 899 patients in auto-auto and 439 in auto-allo. Median follow up of survivors was 118.5 months. Median overall survival (OS) was 78.0 months in auto-auto and 98.3 months in auto-allo (HR = 0.84, P = 0.02). OS was 36.4% vs. 44.1% at 10 years (P = 0.01) for auto-auto and auto-allo, respectively. Progression-free survival was also improved in auto-allo (HR = 0.84, P = 0.004). Risk of non-relapse mortality was higher in auto-allo (10 year 8.3% vs. 19.7%, P < 0.001), while risk of disease progression was higher in auto-auto (10 year 77.2% vs. 61.6%, P < 0.001). Median post relapse survival was 41.5 months in auto-auto and 62.3 months in auto-allo (HR = 0.71, P < 0.001). This supports the existence of durable GVM effect enhancing myeloma control with subsequent therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–8.
Lokhorst HM, Schattenberg A, Cornelissen JJ, van Oers MH, Fibbe W, Russell I, et al. Donor lymphocyte infusions for relapsed multiple myeloma after allogeneic stem-cell transplantation: predictive factors for response and long-term outcome. J Clin Oncol. 2000;18:3031–7.
Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, et al. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4.
Aschan J, Lonnqvist B, Ringden O, Kumlien G, Gahrton G. Graft-versus-myeloma effect. Lancet. 1996;348:346.
Barlogie B, Kyle RA, Anderson KC, Greipp PR, Lazarus HM, Hurd DD, et al. Standard chemotherapy compared with high-dose chemoradiotherapy for multiple myeloma: final results of phase III US Intergroup Trial S9321. J Clin Oncol. 2006;24:929–36.
Gahrton G, Tura S, Ljungman P, Belanger C, Brandt L, Cavo M, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med. 1991;325:1267–73.
Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Bjorkstrand B, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983-93 and 1994-8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001;113:209–16.
Armeson KE, Hill EG, Costa LJ. Tandem autologous vs autologous plus reduced intensity allogeneic transplantation in the upfront management of multiple myeloma: meta-analysis of trials with biological assignment. Bone Marrow Transplant. 2013;48:562–7.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9.
Krishnan A, Pasquini MC, Logan B, Stadtmauer EA, Vesole DH, Alyea E 3rd, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–203.
Gahrton G, Iacobelli S, Bjorkstrand B, Hegenbart U, Gruber A, Greinix H, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–63.
Giaccone L, Storer B, Patriarca F, Rotta M, Sorasio R, Allione B, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. 2011;117:6721–7.
Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011;29:3016–22.
Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008;112:3591–3.
Moreau P, Garban F, Attal M, Michallet M, Marit G, Hulin C, et al. Long-term follow-up results of IFM99-03 and IFM99-04 trials comparing nonmyeloablative allotransplantation with autologous transplantation in high-risk de novo multiple myeloma. Blood. 2008;112:3914–5.
Garban F, Attal M, Michallet M, Hulin C, Bourhis JH, Yakoub-Agha I, et al. Prospective comparison of autologous stem cell transplantation followed by dose-reduced allograft (IFM99-03 trial) with tandem autologous stem cell transplantation (IFM99-04 trial) in high-risk de novo multiple myeloma. Blood. 2006;107:3474–80.
Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N. Engl J Med. 2007;356:1110–20.
Giralt S, Costa LJ, Maloney D, Krishnan A, Fei M, Antin JH, et al. Tandem autologous-autologous versus autologous-allogeneic hematopoietic stem cell transplant for patients with multiple myeloma: long-term follow-up results from the blood and marrow transplant clinical trials network 0102 trial. Biol Blood Marrow Transplant. 2019; pii: S1083-8791(19)30785-2. https://doi.org/10.1016/j.bbmt.2019.11.018. [Epub ahead of print].
Htut M, D’Souza A, Krishnan A, Bruno B, Zhang MJ, Fei M, et al. Autologous/allogeneic hematopoietic cell transplantation versus tandem autologous transplantation for multiple myeloma: comparison of long-term postrelapse survival. Biol Blood Marrow Transplant. 2018;24:478–85.
Maffini E, Storer BE, Sandmaier BM, Bruno B, Sahebi F, Shizuru JA, et al. Long-term follow up of tandem autologous-allogeneic hematopoietic cell transplantation for multiple myeloma. Haematologica. 2019;104:380–91.
Giaccone L, Evangelista A, Patriarca F, Sorasio R, Pini M, Carnevale-Schianca F, et al. Impact of new drugs on the long-term follow-up of upfront tandem autograft-allograft in multiple myeloma. Biol Blood Marrow Transplant. 2018;24:189–93.
Kneppers E, van der Holt B, Kersten MJ, Zweegman S, Meijer E, Huls G, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118:2413–9.
Kroger N, Zabelina T, Klyuchnikov E, Kropff M, Pfluger KH, Burchert A, et al. Toxicity-reduced, myeloablative allograft followed by lenalidomide maintenance as salvage therapy for refractory/relapsed myeloma patients. Bone Marrow Transplant. 2013;48:403–7.
Wolschke C, Stubig T, Hegenbart U, Schonland S, Heinzelmann M, Hildebrandt Y, et al. Postallograft lenalidomide induces strong NK cell-mediated antimyeloma activity and risk for T cell-mediated GvHD: results from a phase I/II dose-finding study. Exp Hematol. 2013;41:134–42. e133.
Alsina M, Becker PS, Zhong X, Adams A, Hari P, Rowley S, et al. Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1183–9.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–21.
Ladetto M, Ferrero S, Drandi D, Festuccia M, Patriarca F, Mordini N, et al. Prospective molecular monitoring of minimal residual disease after non-myeloablative allografting in newly diagnosed multiple myeloma. Leukemia. 2016;30:1211–4.
Moreau P, Hullin C, Garban F, Yakoub-Agha I, Benboubker L, Attal M, et al. Tandem autologous stem cell transplantation in high-risk de novo multiple myeloma: final results of the prospective and randomized IFM 99-04 protocol. Blood. 2006;107:397–403.
Lokhorst HM, van der Holt B, Cornelissen JJ, Kersten MJ, van Oers M, Raymakers R, et al. Donor versus no-donor comparison of newly diagnosed myeloma patients included in the HOVON-50 multiple myeloma study. Blood. 2012;119:6219–25. quiz 6399
Ghosh N, Ye X, Tsai HL, Bolanos-Meade J, Fuchs EJ, Luznik L, et al. Allogeneic blood or marrow transplantation with post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis in multiple myeloma. Biol Blood Marrow Transplant. 2017;23:1903–9.
Acknowledgements
This work was presented as an oral abstract at the 61st Annual Meeting of the American Society of Hematology, Orlando, FL, USA, December 7–10, 2019. This study was not funded commercially. Support for this study was provided to the Blood and Marrow Transplant Clinical Trials Network by grant #U01HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, along with funding from the Southwest Oncology Group (SWOG grant award U10CA180888). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or SWOG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LJC: Consultancy (Karyopharm, Celgene, Amgen, Sanofi, Abbvie, GSK), Research Funding (Amgen, Janssen); Honoraria (Celgene, Janssen, Amgen). MCP Consultancy (Pfizer, Medigene, Amgen, Novartis), Research Funding (Kite Pharmaceuticals, BMS). JB: Advisory committee (Janssen, Celgene, Amgen, Takeda). SS Research Funding (Prothena, Takeda, Janssen), Honoraria (Prothena, Medac, Janssen). PH: Consultancy (Celgene, Takeda, BMS, Janssen, Kite Pharmaceuticals, Amgen, Spectrum, Abbvie), Honoraria (Celgene, Takeda, Janssen, Kite Pharmaceuticals, Sanofi, Abbvie), Research Funding (Celgene, Takeda, BMS, Kite Pharmaceuticals, Spectrum). SAG: Consultancy (Jazz Pharmaceuticals, Novartis, Takeda, Celgene, Kite Pharmaceuticals, Johnson & Johnson, Actinium, Amgen, Spectrum Pharmaceuticals), Research Funding (Takeda, Celgene, Johnson & Johnson, Actinium, Miltenyi, Amgen). FP: Membership on Advisory Committee (Takeda, Celgene, Janssen). AYK: Consultancy (Abbvie, Novartis, Celgene, Takeda), Research Funding (Abbvie, Novartis, Tmunity). GG: Consultancy (Fujimoto Pharmaceutical Corporation). The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Costa, L.J., Iacobelli, S., Pasquini, M.C. et al. Long-term survival of 1338 MM patients treated with tandem autologous vs. autologous-allogeneic transplantation. Bone Marrow Transplant 55, 1810–1816 (2020). https://doi.org/10.1038/s41409-020-0887-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-0887-4
This article is cited by
-
Bridging advanced myeloma patients to subsequent treatments and clinical trials with classical chemotherapy and stem cell support
Bone Marrow Transplantation (2023)
-
Cellular Immunotherapies for Multiple Myeloma: Current Status, Challenges, and Future Directions
Oncology and Therapy (2022)
-
Current Role of Allogeneic Stem Cell Transplantation in Multiple Myeloma
Oncology and Therapy (2022)
-
Outcomes in newly diagnosed young or high-risk myeloma patients receiving tandem autologous/allogeneic transplant followed by bortezomib maintenance: a phase II study
Bone Marrow Transplantation (2022)
-
Evaluation of EuroFlow minimal residual disease measurement and donor chimerism monitoring following tandem auto-allogeneic transplantation for multiple myeloma
Bone Marrow Transplantation (2021)