Abstract
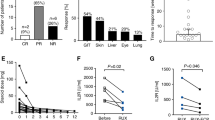
Steroids remain the initial therapy for acute graft-vs.-host disease (AGVHD). Strategies to improve response and minimize steroid exposure are needed. We report results of a randomized, adaptive, Bayesian-designed, phase II trial of prednisone with or without extracorporeal photopheresis (ECP) as an initial therapy for patients with newly diagnosed AGVHD. The primary endpoint was success at day 56 defined as: alive, in remission, achieving AGVHD response without additional therapy, and on <1 mg/kg at day 28 and <0.5 mg/kg on day 56 of steroids. Eighty-one patients were randomized to the ECP arm (n = 51) or steroids alone (n = 30). Median age was 54 years (range: 17–75); 90% had grade II AGVHD and 10% had grades III and IV AGVHD, with skin (85%), upper (22%)/lower (22%) gastrointestinal, and liver (10%) involvement. The ECP arm had a higher probability of success (0.815) and exceeded the predefined threshold for determining the investigational arm promising. ECP was potentially more beneficial than steroids-alone in skin-only AGVHD (response rate: 72% vs. 57%, respectively) than for visceral-organ AGVHD (47% vs. 43%, respectively). The addition of ECP to steroids may result in higher GVHD response as initial therapy for AGVHD, especially for patients with skin-only involvement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
MacMillan ML, DeFor TE, Holtan SG, Rashidi A, Blazar BR, Weisdorf DJ Validation of Minnesota acute graft-versus-host disease risk score. Haematologica. 2020:105:519–24.
Levine JE, Braun TM, Harris AC, Holler E, Taylor A, Miller H, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2:e21–9.
MacMillan ML, Robin M, Harris AC, DeFor TE, Martin PJ, Alousi A, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21:761–7.
Antin JH, Chen AR, Couriel DR, Ho VT, Nash RA, Weisdorf D. Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:655–68.
Weisdorf D, Haake R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–30.
Bolanos-Meade J, Logan BR, Alousi AM, Antin JH, Barowski K, Carter SL, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124:3221–7.
Couriel DR, Saliba R, de Lima M, Giralt S, Andersson B, Khouri I, et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1555–62.
Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–7.
Lee SJ, Zahrieh D, Agura E, MacMillan ML, Maziarz RT, McCarthy PL Jr., et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–64.
Cragg L, Blazar BR, Defor T, Kolatker N, Miller W, Kersey J, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–7.
Cahn JY, Bordigoni P, Tiberghien P, Milpied N, Brion A, Widjenes J, et al. Treatment of acute graft-versus-host disease with methylprednisolone and cyclosporine with or without an anti-interleukin-2 receptor monoclonal antibody. A multicenter phase III study. Transplantation. 1995;60:939–42.
Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15:457–65.
Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303.
Maeda A, Schwarz A, Bullinger A, Morita A, Peritt D, Schwarz T. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181:5956–62.
Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–21.
Di Renzo M, Sbano P, De Aloe G, Pasqui AL, Rubegni P, Ghezzi A, et al. Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients. Clin Exp Immunol. 2008;151:407–13.
Peritt D. Potential mechanisms of photopheresis in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:7–12.
Lamioni A, Parisi F, Isacchi G, Giorda E, Di Cesare S, Landolfo A, et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–50.
Greinix HT, van Besien K, Elmaagacli AH, Hillen U, Grigg A, Knobler R, et al. Progressive improvement in cutaneous and extracutaneous chronic graft-versus-host disease after a 24-week course of extracorporeal photopheresis-results of a crossover randomized study. Biol Blood Marrow Transplant. 2011;17:1775–82.
Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–74.
Sakellari I, Gavriilaki E, Batsis I, Mallouri D, Panteliadou AK, Lazaridou A, et al. Favorable impact of extracorporeal photopheresis in acute and chronic graft versus host disease: prospective single-center study. J Clin Apher. 2018;33:654–60.
Perotti C, Del Fante C, Tinelli C, Viarengo G, Scudeller L, Zecca M, et al. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfus. 2010;50:1359–69.
Greinix HT, Knobler RM, Worel N, Schneider B, Schneeberger A, Hoecker P, et al. The effect of intensified extracorporeal photochemotherapy on long-term survival in patients with severe acute graft-versus-host disease. Haematologica. 2006;91:405–8.
Messina C, Locatelli F, Lanino E, Uderzo C, Zacchello G, Cesaro S, et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br J Haematol. 2003;122:118–27.
Salvaneschi L, Perotti C, Zecca M, Bernuzzi S, Viarengo G, Giorgiani G, et al. Extracorporeal photochemotherapy for treatment of acute and chronic GVHD in childhood. Transfus. 2001;41:1299–305.
Cid J, Carbasse G, Suarez-Lledo M, Moreno DF, Martinez C, Gutierrez-Garcia G, et al. Efficacy and safety of one-day offline extracorporeal photopheresis schedule processing one total blood volume for treating patients with graft-versus-host disease. Transfus. 2019;59:2636–42.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–8.
Acknowledgements
This physician-initiated trial was supported by a grant from Therakos, Inc., a Mallinckrodt Pharmaceuticals company. Additional support was provided through the Cancer Center Support Grant (NCI Grant P30 CA016672).
Author information
Authors and Affiliations
Contributions
RSM contributed patient enrollment, paper writing, and interpretation of results; RB contributed to trial design, interpretation of results, and statistical analysis; GR contributed data collection and interpretation of results; BJO contributed data collection; URP, CMH, MQ, PA, IFK, BO, SOC, and EJS contributed patient enrollment and interpretation of results; KR contributed patient enrollment, laboratory analysis, and interpretation of results; DM and KK contributed laboratory analysis and interpretation of results; DRC contributed study design; REC contributed to study design, patient enrollment, and interpretation of results; and AMA contributed to study design, enrollment of patients on study, interpretation of results, and wrote the paper. All authors reviewed and approved the paper.
Corresponding author
Ethics declarations
Conflict of interest
AMA has served as a speaker for Therakos, Inc. Other authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Mehta, R.S., Bassett, R., Rondon, G. et al. Randomized phase II trial of extracorporeal phototherapy and steroids vs. steroids alone for newly diagnosed acute GVHD. Bone Marrow Transplant 56, 1316–1324 (2021). https://doi.org/10.1038/s41409-020-01188-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01188-4
This article is cited by
-
Extracorporeal photopheresis (ECP) improves overall survival in the treatment of steroid refractory acute graft-versus-host disease (SR aGvHD)
Bone Marrow Transplantation (2023)
-
Extracorporeal photopheresis is catching up the pole position
Bone Marrow Transplantation (2021)