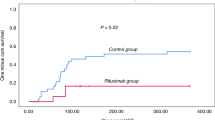
Abstract
Hematopoietic stem cell transplantation (HCT) has been increasingly used for patients with inherited metabolic disorders (IMD). Immune mediated cytopenias (IMCs) after HCT, manifesting as hemolytic anemia, thrombocytopenia, and/or neutropenia, are recognized as a significant complication in this patient population, yet our understanding of the incidence, risk factors, and pathophysiology is currently limited. Review of the published literature demonstrates a higher incidence in younger patients who undergo HCT for a nonmalignant disease indication. However, a few reports suggest that the incidence is even higher among those with IMD (incidence ranging from 10 to 56%). This review summarizes the literature, provides an approach to better understanding of the possible etiology of IMCs, and proposes a diagnostic and management plan for patients with IMD who develop single or multi-lineage cytopenias after HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chen FE, Owen I, Savage D, Roberts I, Apperley J, Goldman JM, et al. Late onset haemolysis and red cell autoimmunisation after allogeneic bone marrow transplant. Bone Marrow Transplant. 1997;19:491–5.
Dovat S, Roberts RL, Wakim M, Stiehm ER, Feig SA. Immune thrombocytopenia after umbilical cord progenitor cell transplant: response to vincristine. Bone Marrow Transplant. 1999;24:321–3.
Horn B, Viele M, Mentzer W, Mogck N, DeSantes K, Cowan M. Autoimmune hemolytic anemia in patients with SCID after T cell-depleted BM and PBSC transplantation. Bone Marrow Transplant. 1999;24:1009–13.
Bhatt V, Shune L, Lauer E, Lubin M, Devlin SM, Scaradavou A, et al. Autoimmune hemolysis and immune thrombocytopenic purpura after cord blood transplantation may be life-threatening and warrants early therapy with rituximab. Bone Marrow Transplant. 2016;51:1579–83.
Chang TY, Jaing TH, Wen YC, Huang IA, Chen SH, Tsay PK. Risk factor analysis of autoimmune hemolytic anemia after allogeneic hematopoietic stem cell transplantation in children. Medicine. 2016;95:e5396.
Daikeler T, Labopin M, Ruggeri A, Crotta A, Abinun M, Hussein AA, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the Autoimmune Disease Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2013;121:1059–64.
Faraci M, Zecca M, Pillon M, Rovelli A, Menconi MC, Ripaldi M, et al. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant. 2014;20:272–8.
Holbro A, Abinun M, Daikeler T. Management of autoimmune diseases after haematopoietic stem cell transplantation. Br J Haematol. 2012;157:281–90.
Kruizinga MD, van Tol MJD, Bekker V, Netelenbos T, Smiers FJ, Bresters D, et al. Risk factors, treatment, and immune dysregulation in autoimmune cytopenia after allogeneic hematopoietic stem cell transplantation in pediatric patients. Biol Blood Marrow Transplant. 2018;24:772–8.
O’Brien TA, Eastlund T, Peters C, Neglia JP, Defor T, Ramsay NK, et al. Autoimmune haemolytic anaemia complicating haematopoietic cell transplantation in paediatric patients: high incidence and significant mortality in unrelated donor transplants for non-malignant diseases. Br J Haematol. 2004;127:67–75.
Page KM, Mendizabal AM, Prasad VK, Martin PL, Parikh S, Wood S, et al. Posttransplant autoimmune hemolytic anemia and other autoimmune cytopenias are increased in very young infants undergoing unrelated donor umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2008;14:1108–17.
Deambrosis D, Lum SH, Hum RM, Poulton K, Ogden W, Jones S, et al. Immune cytopenia post-cord transplant in Hurler syndrome is a forme fruste of graft rejection. Blood Adv. 2019;3:570–4.
Gupta A, Downey M, Shanley R, Jennissen C, Miller WP, Lund TC, et al. Reduced-toxicity (BuFlu) conditioning is better tolerated but has a higher second transplantation rate compared to myeloablative conditioning (BuCy) in children with inherited metabolic disorders. Biol Blood Marrow Transplant. 2020;26:486–92.
Szanto CL, Langenhorst J, de Koning C, Nierkens S, Bierings M, Huitema ADR, et al. Predictors for autoimmune cytopenias after allogeneic hematopoietic cell transplantation in children. Biol Blood Marrow Transplant. 2020;26:114–22.
Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20:349–60.
Ahmed I, Teruya J, Murray-Krezan C, Krance R. The incidence of autoimmune hemolytic anemia in pediatric hematopoietic stem cell recipients post-first and post-second hematopoietic stem cell transplant. Pediatr Transplant. 2015;19:391–8.
Hwang-Bo S, Kim SK, Lee JW, Jang PS, Chung NG, Jeong DC, et al. Treatment and response of autoimmune cytopenia occurring after allogeneic hematopoietic cell transplantation in children. Blood Res. 2017;52:119–24.
Scordo SM, Shah GL, Kosuri S, Adrianzen Herrera DA, Hsu M, Devlin SM, et al. Immune-mediated hemolytic anemia (IMHA) and immune thrombocytopenia (ITP) after ex-vivo CD34+ selected allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2017;23:S129–30.
Lum SH, Selvarajah S, Deya-Martinez AA, McNaughton P, Sobh A, Waugh S, et al. Outcome of autoimmune cytopenia after hematopoietic cell transplantation in primary immunodeficiency. J Allergy Clin Immunol. 2020;146:P406–16.
Griesemer AD, Sorenson EC, Hardy MA. The role of the thymus in tolerance. Transplantation. 2010;90:465–74.
Takahama Y, Nitta T, Mat Ripen A, Nitta S, Murata S, Tanaka K. Role of thymic cortex-specific self-peptides in positive selection of T cells. Semin Immunol. 2010;22:287–93.
Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–10.
Chapoval S, Dasgupta P, Dorsey NJ, Keegan AD. Regulation of the T helper cell type 2 (Th2)/T regulatory cell (Treg) balance by IL-4 and STAT6. J Leukoc Biol. 2010;87:1011–8.
King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77.
Roord ST, de Jager W, Boon L, Wulffraat N, Martens A, Prakken B, et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111:5233–41.
Seidel MG, Fritsch G, Lion T, Jurgens B, Heitger A, Bacchetta R, et al. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113:5689–91.
Archer LD, Langford-Smith KJ, Bigger BW, Fildes JE. Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity. J Inherit Metab Dis. 2014;37:1–12.
Dani A, Chaudhry A, Mukherjee P, Rajagopal D, Bhatia S, George A, et al. The pathway for MHCII-mediated presentation of endogenous proteins involves peptide transport to the endo-lysosomal compartment. J Cell Sci. 2004;117:4219–30.
Rigante D, Cipolla C, Basile U, Gulli F, Savastano MC. Overview of immune abnormalities in lysosomal storage disorders. Immunol Lett. 2017;188:79–85.
Neely JA, Dvorak CC, Pantell MS, Melton A, Huang JN, Shimano KA. Autoimmune cytopenias in pediatric hematopoietic cell transplant patients. Front Pediatr. 2019;7:171.
Crowther M, Chan YL, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence-based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036–40.
Hartert A, Willenbacher W, Gunzelmann S, Roemer E, Basara N, Fauser AA, et al. Successful treatment of thrombocytopenia and hemolytic anemia with IvIG in a patient with lupus-like syndrome after mismatched related PBSCT. Bone Marrow Transplant. 2001;27:337–40.
Khandelwal P, Davies SM, Grimley MS, Jordan MB, Curtis BR, Jodele S, et al. Bortezomib for refractory autoimmunity in pediatrics. Biol Blood Marrow Transplant. 2014;20:1654–9.
Mehta B, Mahadeo K, Zaw R, Tang S, Kapoor N, Abdel-Azim H. Bortezomib for effective treatment of a child with refractory autoimmune hemolytic anemia post allogeneic hematopoietic stem cell transplant. Pediatr Blood Cancer. 2014;61:2324–5.
Ratnasingam S, Walker PA, Tran H, Kaplan ZS, McFadyen JD, Tran H, et al. Bortezomib-based antibody depletion for refractory autoimmune hematological diseases. Blood Adv. 2016;1:31–5.
Bride KL, Vincent T, Smith-Whitley K, Lambert MP, Bleesing JJ, Seif AE, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016;127:17–28.
O’Connell N, Goodyer M, Gleeson M, Storey L, Williams M, Cotter M, et al. Successful treatment with rituximab and mycophenolate mofetil of refractory autoimmune hemolytic anemia post-hematopoietic stem cell transplant for dyskeratosis congenita due to TINF2 mutation. Pediatr Transplant. 2014;18:E22–4.
Bowen DA, Call TG, Shanafelt TD, Kay NE, Schwager SM, Reinalda MS, et al. Treatment of autoimmune cytopenia complicating progressive chronic lymphocytic leukemia/small lymphocytic lymphoma with rituximab, cyclophosphamide, vincristine, and prednisone. Leuk Lymphoma. 2010;51:620–7.
Waespe N, Zeilhofer U, Gungor T. Treatment-refractory multi-lineage autoimmune cytopenia after unrelated cord blood transplantation: remission after combined bortezomib and vincristine treatment. Pediatr Blood Cancer. 2014;61:2112–4.
Blennerhassett R, Sudini L, Gottlieb D, Bhattacharyya A. Post-allogeneic transplant Evans syndrome successfully treated with daratumumab. Br J Haematol. 2019;187:e48–51.
Even-Or E, Naser Eddin A, Shadur B, Dinur Schejter Y, Najajreh M, Zelig O, et al. Successful treatment with daratumumab for post-HSCT refractory hemolytic anemia. Pediatr Blood Cancer. 2020;67:e28010.
Schuetz C, Hoenig M, Moshous D, Weinstock C, Castelle M, Bendavid M, et al. Daratumumab in life-threatening autoimmune hemolytic anemia following hematopoietic stem cell transplantation. Blood Adv 2018;2:2550–3.
Zhai J, Ding M, Yang T, Zuo B, Weng Z, Zhao Y, et al. Flow cytometric immunobead assay for quantitative detection of platelet autoantibodies in immune thrombocytopenia patients. J Transl Med. 2017;15:214.
Zhao Y, Zhu M, Jiang M, Zuo B, Wu Q, Ruan C, et al. An improved flow cytometric immunobead array to detect autoantibodies in plasma from patients with immune thrombocytopenic purpura. Clin Chim. 2015;438:396–400.
Porretti L, Farruggia P, Colombo FS, Cattaneo A, Ghilardi R, Mirra N, et al. Diagnostic value of cell bound and circulating neutrophil antibody detection in pediatric neutropenia. Pediatr Blood Cancer. 2018;65:65:e26904.
Salas MQ, Alahmari A, Lipton JH. Successful treatment of refractory red cell aplasia after allogeneic hematopoietic cell transplantation with daratumumab. Eur J Haematol. 2020;104:145–7.
Pavlasova G, Mraz M. The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica. 2020;105:1494–506.
Leibler C, Thiolat A, Henique C, Samson C, Pilon C, Tamagne M, et al. Control of humoral response in renal transplantation by belatacept depends on a direct effect on B cells and impaired T follicular helper-B cell crosstalk. J Am Soc Nephrol. 2018;29:1049–62.
Acknowledgements
This expert consensus was based on information developed for and during a meeting supported by Magenta Therapeutics. The content of this paper is solely that of the authors. Beth Kamp, PharmD, a medical writer supported by funding from Magenta Therapeutics provided initial editorial assistance to the authors during preparation of this paper.
Author information
Authors and Affiliations
Contributions
AG, JB, CE, TL, PO, AS, JW, and RW provided review of the literature and contributed to the development of the content of the paper. BB provided critical input regarding pathophysiology of IMC and critical review of paper. AG wrote the paper with contributions from all authors.
Corresponding author
Ethics declarations
Conflict of interest
This expert consensus was based on information developed for and during a meeting supported by Magenta Therapeutics and subsequent data and literature review along with discussions. The content of this paper is solely that of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gupta, A.O., Jan Boelens, J., Ebens, C.L. et al. Consensus opinion on immune-mediated cytopenias after hematopoietic cell transplant for inherited metabolic disorders. Bone Marrow Transplant 56, 1238–1247 (2021). https://doi.org/10.1038/s41409-020-01179-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-020-01179-5
This article is cited by
-
Immune-mediated cytopenias (IMCs) after HSCT for pediatric non-malignant disorders: epidemiology, risk factors, pathogenesis, and treatment
European Journal of Pediatrics (2023)
-
B-cell depletion abrogates immune mediated cytopenia and rejection of cord blood transplantation in Hurler syndrome
Bone Marrow Transplantation (2022)
-
Allogeneic hematopoietic stem cell transplantation for inherited metabolic disorders
International Journal of Hematology (2022)