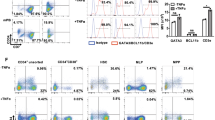
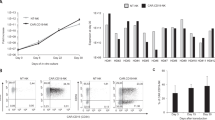
Abstract
Prolonged T-cell immunodeficiency following HLA- incompatible hematopoietic stem cell transplantation (HSCT) represents a major obstacle hampering the more widespread use of this approach. Strategies to fasten T-cell reconstitution in this setting are highly warranted as opportunistic infections and an increased risk of relapse account for high rates of morbidity and mortality especially during early month following this type of HSCT. We have implemented a feeder free cell system based on the use of the notch ligand DL4 and cytokines allowing for the in vitro differentiation of human T-Lymphoid Progenitor cells (HTLPs) from various sources of CD34+ hematopoietic stem and precursor cells (HSPCs). Co- transplantion of human T-lymphoid progenitors (HTLPs) and non- manipulated HSPCs into immunodeficient mice successfully accelerated the reconstitution of a polyclonal T-cell repertoire. This review summarizes preclinical data on the use of T-cell progenitors for treatment of post- transplantation immunodeficiency and gives insights into the development of GMP based protocols for potential clinical applications including gene therapy approaches. Future clinical trials implementing this protocol will aim at the acceleration of immune reconstitution in different clinical settings such as SCID and leukemia patients undergoing allogeneic transplantation. Apart from pure cell-therapy approaches, the combination of DL-4 culture with gene transduction protocols will open new perspectives in terms of gene therapy applications for primary immunodeficiencies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Change history
16 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41409-022-01629-2
References
Reisner Y, Itzicovitch LEA, Meshorer A, Sharon N. Hemopoietic stem cell transplant using mouse bone marrow spleen cells fractionated lectins. Proc Natl Acad Sci USA. 1978;75:2933–6.
Urbano-Ispizua A, Rozman C, Martínez C, Marín P, Briones J, Rovira M, et al. Rapid engraftment without significant graft-versus-host disease after allogeneic transplantation of CD34 + selected cells from peripheral blood. Blood. 1997;89:3967–73.
Robinson TM, O’Donnell PV, Fuchs EJ, Luznik L. Haploidentical bone marrow and stem cell transplantation: experience with post-transplantation cyclophosphamide. Semin Hematol 2016;53:90–7.
Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: Never say never again. Tissue Antigens. 2012;79:83–9.
Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol 2007;19:318–30.
Krenger W, Blazar BR, Hollander GA. Review article Thymic T-cell development in allogeneic stem cell transplant. Blood 2011;117:6768–76.
Heimall J, Logan BR, Cowan MJ, Notarangelo LD, Griffith LM, Puck JM, et al. Immune reconstitution and survival of 100 SCID patients post-hematopoietic cell transplant: A PIDTC natural history study. Blood. 2017;130:2718–27.
Touzot F, Moshous D, Creidy R, Neven B, Frange P, Cros G, et al. Faster T-cell development following gene therapy compared to haplo-identical hematopoietic stem cell transplantation in the treatment of SCID-X1. Blood. 2015;125:1–8.
Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol 2008;30:425–37.
Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol 2012;19:324–55.
Reimann C, Dal Cortivo L, Hacein-Bey-Abina S, Fischer A, André-Schmutz I, Cavazzana-Calvo M. Advances in adoptive immunotherapy to accelerate T-cellular immune reconstitution after HLA-incompatible hematopoietic stem cell transplantation. Immunotherapy. 2010;2:481–96.
Clave E, Lisini D, Douay C, Giorgiani G, Busson M, Zecca M, et al. Thymic function recovery after unrelated donor cord blood or T-cell depleted HLA-haploidentical stem cell transplantation correlates with leukemia relapse. Front Immunol 2013;4:1–8.
Cavazzana M, Six E, Lagresle-Peyrou C, André-Schmutz I, Hacein-Bey-Abina S. Gene therapy for X-linked severe combined immunodeficiency: where do we stand? Hum Gene Ther 2016;27:108–16.
Hazenberg MD1, Otto SA, de Pauw ES, Roelofs H, Fibbe WE, Hamann D, et al. T-cell receptor excision circle and T-cell dynamics after allogeneic stem cell transplantation are related to clinical events. 2002;99:3449–53.
Clave E, Busson M, Douay C, Peffault de Latour R, Berrou J, Rabian C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2011;113:6477–84.
Chaudhry MS, Velardi E, Malard F, van den Brink MR. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: time to T up the thymus. J Immunol 2017;198:40–6.
Krenger W, Rossi S, Holländer GA. Apoptosis of thymocytes during acute graft-versus-host disease is independent of glucocorticoids. Transplantation. 2000;69:2190–3.
Wang SD, Huang KJ, Lin YS, Lei HY. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–21.
Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol 2006;177:169–76.
Mocarski ES, Bonyhadif M, Salimif S, McCune JM, Kaneshima H. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Med Sci 1993;90:104–8.
Koning C, de, Admiraal R, Nierkens S, Boelens JJ. Human herpesvirus 6 viremia affects T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Blood Adv 2018;2:428–32.
Svaldi M, Lanthaler AJ, Dugas M, Lohse P, Pescosta N, Straka C, et al. T-cell receptor excision circles: a novel prognostic parameter for the outcome of transplantation in multiple myeloma patients. Br J Haematol 2003;122:795–801.
Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med 2008;205:2515–23.
Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med 2008;205:2507–13.
Taghon T, Waegemans E, Van de Walle I. Notch signaling during human T cell development. Curr Top Microbiol Immunol. 2012;360:75–97.
Hosokawa H, Rothenberg EV. Cytokines, Transcription Factors, and the Initiation of T-cell development. Cold Spring Harb Perspect Biol. 2018;1:1–20.
Six EM, Benjelloun F, Garrigue A, Bonhomme D, Morillon E, Rouiller J, et al. Cytokines and culture medium have a major impact on human in vitro T-cell differentiation. Blood Cells Mol Dis 2011;47:72–8.
Schmitt TM, Zúñiga-Pflücker JC. Thymus-derived signals regulate early T-cell development. Crit Rev Immunol 2005;25:141–59.
Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 2010;16:232–6.
Reimann C, Six E, Dal-Cortivo L, Schiavo A, Appourchaux K, Lagresle-Peyrou C, et al. Human T-lymphoid progenitors generated in a feeder-cell-free delta-like-4 culture system promote T-cell reconstitution in NOD/SCID/γc-/-mice. Stem Cells. 2012;30:1771–80.
Six EM, Bonhomme D, Monteiro M, Beldjord K, Jurkowska M, Cordier-Garcia C, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med 2007;204:3085–93.
Simons L, Ma K, de Chappedelaine C, Elkaim E, Olivré J, Susini S, et al. Generation of adult human T-cell progenitors for immunotherapeutic applications. J Allergy Clin Immunol 2018;141:1491–1494.e4.
Hacein-Bey-Abina S, Pai S-Y, Gaspar HB, Armant M, Berry CC, Blanche S, et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N Engl J Med 2014;371:1407–17.
Bernadin O, Amirache F, Girard-Gagnepain A, Moirangthem RD, Lévy C, Ma K et al. Baboon envelope LVs efficiently transduced human adult, fetal, and progenitor T cells and corrected SCID-X1 T-cell deficiency. Blood Adv. 2019;3:461–75.
Acknowledgements
We thank Pauline Huguenin for help in bibliography.
Funding
The study was funded by the French National Institute of Health and Medical Research (INSERM), a European Research Council grant (ERC Regenerative Therapy, 269037), a European Union FP7 grant (CELL-PID, 261387), a European Union H2020 grant (SCIDNet, 666908), Imagine Institute, a Clinical Research Hospital Program (PHRC) (Ministry of Health and Social Affairs), an INCA-Plan Cancer grant (2009–2013) and a public grant overseen by the French National Research Agency (ANR) as part of the program “Investissements d’Avenir” (reference: ANR-10-IAHU-01). LS was funded by Imagine Institute. KM was funded by a China Scholarship Council (CSC). EE was funded by an INSERM-Plan Cancer fellowship. CR was funded by postdoctoral scholarships from the Deutsche Forschungsgemeinschaft (DFG). TT was funded by the Fund for Scientific Research Flanders (FWO Vlaanderen research projects G0B2913N). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Publication of this supplement was sponsored by Gilead Sciences Europe Ltd, Cell Source, Inc., The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MC and IA-S own equity in Smart Immune and hold two patents in this area, about the in vitro process of production of T-cell progenitors. KM is co-inventor of patent WO 2018/146297 A1, Methods for generating T-cells progenitors. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
André, I., Simons, L., Ma, K. et al. Ex vivo generated human T-lymphoid progenitors as a tool to accelerate immune reconstitution after partially HLA compatible hematopoietic stem cell transplantation or after gene therapy. Bone Marrow Transplant 54 (Suppl 2), 749–755 (2019). https://doi.org/10.1038/s41409-019-0599-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0599-9