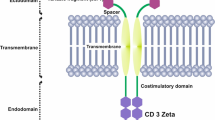
Abstract
One of the remaining and unresolved problems in allogeneic stem cell transplantation, especially following a T cell-depleted transplant as often performed in the setting of haploidentical transplantation, is the relapse of the underlying hematological malignancy. It has been demonstrated that in the last years we have made major progress in controlling infections, acute and chronic GvHD and making stem cell transplantation available to elderly patients. However, little improvement has been made to achieve better tumor control and to lower the relapse rate. Thus, novel immunotherapeutic strategies are increasingly used prior to or even following allogeneic stem cell transplantation to better control the underlying malignancy and thus, to reduce the relapse rate. These novel immunotherapeutic strategies comprise monoclonal antibodies, immunotoxins and even more effective T cell redirecting strategies like bispecific antibodies and T cells transduced with either chimeric antigen receptors (CAR) or (affinity-tuned) T cell receptors (TCR).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Dahlén E, Veitonmäki N, Norlén P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccin Immunother. 2018;6:3–17.
Yuraszeck T, Kasichayanula S, Benjamin JE. Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin Pharm Ther. 2017;101:634–45.
Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–7.
Topp MS, Gökbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–40.
Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a Phase I study. J Clin Oncol. 2016;34:1104–11.
Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66.
von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–9.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
Boucher K, Parquet N, Widen R, Shain K, Baz R, Alsina M, et al. Stemness of B-cell progenitors in multiple myeloma bone marrow. Clin Cancer Res. 2012;18:6155–68.
Kantarjian HM, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera J-M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47.
Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematol Am Soc Hematol Educ Program. 2016;2016:567–72.
Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9.
Brandl C, Haas C, d’Argouges S, Fisch T, Kufer P, Brischwein K, et al. The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol Immunother. 2007;56:1551–63.
Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–7.
Topp MS, Zimmerman Z, Cannell P, Dombret H, Maertens J, Stein A, et al. Health-related quality of life in adults with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab. Blood. 2018;131:2906–14.
Jabbour E, Düll J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93:371–4.
Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31:2181–219.
Köhnke T, Krupka C, Tischer J, Knösel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111.
Schuster FR, Stanglmaier M, Woessmann W, Winkler B, Siepermann M, Meisel R, et al. Immunotherapy with the trifunctional anti-CD20 x anti-CD3 antibody FBTA05 (Lymphomun) in paediatric high-risk patients with recurrent CD20-positive B cell malignancies. Br J Haematol. 2015;169:90–102.
Gardner R, Wu D, Cherian S, Fang M, Hanafi LA, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–10.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–95.
Yu H, Sotillo E, Harrington C, Wertheim G, Paessler M, Maude SL, et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol. 2017;92:E11–E13.
Braig F, Brandt A, Goebeler M, Tony HP, Kurze AK, Nollau P, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–4.
Kügler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediated effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150:574–86.
Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–38.
Funding
Publication of this supplement was sponsored by Gilead Sciences Europe Ltd, Cell Source, Inc. The Chorafas Institute for Scientific Exchange of the Weizmann Institute of Science, Kiadis Pharma, Miltenyi Biotec, Celgene, Centro Servizi Congressuali, Almog Diagnostic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
LR has received consulting fees from Pfizer and grant support from DFG. HE has received consulting fees, grants, and honoraria from Amgen, Celgene, Janssen, and BMS. KMK has received consulting fees from Janssen, and Oncopeptide. MST received lecture fees from Amgen, Regeneron, Roche, and lecture fees from Amgen. JD received consulting fees from Gilead.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Einsele, H., Rasche, L., Topp, M.S. et al. The use of bispecific antibodies to optimize the outcome of patients with acute leukemia, lymphoma and multiple myeloma after SCT. Bone Marrow Transplant 54 (Suppl 2), 721–726 (2019). https://doi.org/10.1038/s41409-019-0596-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-019-0596-z
This article is cited by
-
Long-term outcome after allogeneic stem cell transplantation in multiple myeloma
Annals of Hematology (2021)