Abstract
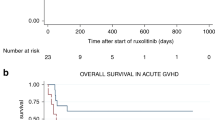
Sclerotic chronic graft vs. host disease (cGVHD) still has a large impact on morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). We performed the first prospective study to test whether sequential therapy of the anti-CD20 antibody rituximab followed by 6 months treatment with tyrosine kinase inhibitor nilotinib is a favorable treatment strategy for patients with sclerotic cGVHD. Twenty-nine patients were included, 24 were available for analysis. We observed objective responses in 71% of patients (two patients CR, 15 patients PR). Moreover, two out of five patients suffering from severe ulcerations showed complete resolution of ulcers. Observed responses lasted until the end of study follow-up. The majority of responding patients could reduce daily corticosteroid dose with more than 50%. Furthermore, CD5+ B-cells are significantly lower (p = 0.007) in responding patients at baseline, proposing a new biomarker predictive for response. In conclusion, sequential treatment of rituximab followed by nilotinib associates with a very high response rate in this difficult to treat patient population. CD5+ B-cells could assist in guiding treatment choices and might be a first step toward more personalized cGVHD treatment. This trial was registered at the Dutch clinical trial registry as NTR1222.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Pasquini MC, Devine S, Mendizabal A, Baden LR, Wingard JR, Lazarus HM, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–201.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med. 2016;374:43–53.
Finke J, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biol Blood Marrow Transplant. 2012;18:1716–26.
Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4:e183–e91.
Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016;51:1431–8.
Cutler C, Miklos D, Kim HT, Treister N, Woo SB, Bienfang D, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–62.
Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15:1005–13.
van Dorp S, Resemann H, te Boome L, Pietersma F, van Baarle D, Gmelig-Meyling F, et al. The immunological phenotype of rituximab-sensitive chronic graft-versus-host disease: a phase II study. Haematologica. 2011;96:1380–4.
Canninga-van Dijk MR, van der Straaten HM, Fijnheer R, Sanders CJ, van den Tweel JG, Verdonck LF. Anti-CD20 monoclonal antibody treatment in 6 patients with therapy-refractory chronic graft-versus-host disease. Blood. 2004;104:2603–6.
Mohty M, Marchetti N, El-Cheikh J, Faucher C, Furst S, Blaise D. Rituximab as salvage therapy for refractory chronic GVHD. Bone Marrow Transplant. 2008;41:909–11.
Magro L, Mohty M, Catteau B, Coiteux V, Chevallier P, Terriou L, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114:719–22.
Magro L, Catteau B, Coiteux V, Bruno B, Jouet JP, Yakoub-Agha I. Efficacy of imatinib mesylate in the treatment of refractory sclerodermatous chronic GVHD. Bone Marrow Transplant. 2008;42:757–60.
Olivieri A, Locatelli F, Zecca M, Sanna A, Cimminiello M, Raimondi R, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114:709–18.
de Masson A, Bouaziz JD, Peffault de Latour R, Wittnebel S, Ribaud P, Rubio MT, et al. Limited efficacy and tolerance of imatinib mesylate in steroid-refractory sclerodermatous chronic GVHD. Blood. 2012;120:5089–90.
Arai S, Pidala J, Pusic I, Chai X, Jaglowski S, Khera N, et al. A randomized phase II crossover study of imatinib or rituximab for cutaneous sclerosis after hematopoietic cell transplantation. Clin Cancer Res. 2015;22:319–27.
Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–41.
Blay JY, von Mehren M. Nilotinib: a novel, selective tyrosine kinase inhibitor. Semin Oncol. 2011;38(Suppl 1):S3–9.
Beyer C, Distler JH, Distler O. Are tyrosine kinase inhibitors promising for the treatment of systemic sclerosis and other fibrotic diseases? Swiss Med Wkly. 2010;140:w13050.
Kariminia A, Holtan SG, Ivison S, Rozmus J, Hebert MJ, Martin PJ, et al. Heterogeneity of chronic graft-versus-host disease biomarkers: association with CXCL10 and CXCR3+ NK cells. Blood. 2016;127:3082–91.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–56.
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401e1.
Pavletic SZ, Martin P, Lee SJ, Mitchell S, Jacobsohn D, Cowen EW, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–66.
Malard F, Labopin M, Yakoub-Agha I, Chantepie S, Guillaume T, Blaise D, et al. Rituximab-based first line treatment for chronic GVHD after allogeneic SCT: results of a phase 2 study. Blood. 2017;130:2186–95.
Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–50.
Lydyard PM, Lamour A, MacKenzie LE, Jamin C, Mageed RA, Youinou P. CD5+ B cells and the immune system. Immunol Lett. 1993;38:159–66.
Bunch DO, McGregor JG, Khandoobhai NB, Aybar LT, Burkart ME, Hu Y, et al. Decreased CD5(+) B cells in active ANCA vasculitis and relapse after rituximab. Clin J Am Soc Nephrol. 2013;8:382–91.
Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29:2062–8.
Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123:3832–42.
Dubovsky JA, Flynn R, Du J, Harrington BK, Zhong Y, Kaffenberger B, et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest. 2014;124:4867–76.
Acknowledgements
We thank the Multiplex Core Facility UMC, Utrecht, for performing multiplex immunoassays; the Flow Cytometry Core Facility UMC, Utrecht, for support in performing FACS assays; Novartis and Roche for kindly providing study medication. This work was further supported in part by grants from the Dutch Cancer Society to LvdW: KWF-UU 2011-5250 and to JK KWF-UU 2010-4669, 2013-6426, 2014-6790, and 2015-7601.
Author contributions
LtB, EM, SvD, and JK designed the study. LvdW and MS performed the experiments. LvdW analyzed and interpreted the data. LvdW and JK wrote the manuscript. LtB, IN, SvD, MvD, and EM interpreted the data. LtB, IN, MS, RR, EP, MdW, NdJ, MB, BB, and EM provided the clinical data and commented on the manuscript. JK supervised the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JK is cofounder and chief scientific officer of GADETA. His work is partly supported by a grant from Novartis; however, Novartis had no part in the design, analysis, or interpretation of the data or the writing of the manuscript.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
van der Wagen, L., te Boome, L., Schiffler, M. et al. Prospective evaluation of sequential treatment of sclerotic chronic graft versus host disease with rituximab and nilotinib. Bone Marrow Transplant 53, 1255–1262 (2018). https://doi.org/10.1038/s41409-018-0158-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-018-0158-9
This article is cited by
-
Tyrosine kinase inhibitor levels matter in treating chronic GVHD
Bone Marrow Transplantation (2019)