Abstract
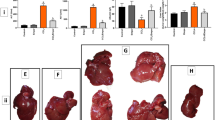
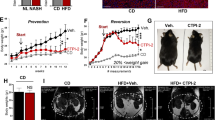
Tacrolimus, one of the macrolide calcineurin inhibitors, is the most frequently used immunosuppressant after transplantation. Long-term administration of tacrolimus leads to dyslipidemia and affects liver lipid metabolism. In this study, we investigated the mode of action and underlying mechanisms of this adverse reaction. Mice were administered tacrolimus (2.5 mg·kg−1·d−1, i.g.) for 10 weeks, then euthanized; the blood samples and liver tissues were collected for analyses. We showed that tacrolimus administration induced significant dyslipidemia and lipid deposition in mouse liver. Dyslipidemia was also observed in heart or kidney transplantation patients treated with tacrolimus. We demonstrated that tacrolimus did not directly induce de novo synthesis of fatty acids, but markedly decreased fatty acid oxidation (FAO) in AML12 cells. Furthermore, we showed that tacrolimus dramatically decreased the expression of HMGCS2, the rate-limiting enzyme of ketogenesis, with decreased ketogenesis in AML12 cells, which was responsible for lipid deposition in normal hepatocytes. Moreover, we revealed that tacrolimus inhibited forkhead box protein O1 (FoxO1) nuclear translocation by promoting FKBP51-FoxO1 complex formation, thus reducing FoxO1 binding to the HMGCS2 promoter and its transcription ability in AML12 cells. The loss of HMGCS2 induced by tacrolimus caused decreased ketogenesis and increased acetyl-CoA accumulation, which promoted mitochondrial protein acetylation, thereby resulting in FAO function inhibition. Liver-specific HMGCS2 overexpression via tail intravenous injection of AAV8-TBG-HMGCS2 construct reversed tacrolimus-induced mitochondrial protein acetylation and FAO inhibition, thus removing the lipid deposition in hepatocytes. Collectively, this study demonstrates a novel mechanism of liver lipid deposition and hyperlipidemia induced by long-term administration of tacrolimus, resulted from the loss of HMGCS2-mediated ketogenesis and subsequent FAO inhibition, providing an alternative target for reversing tacrolimus-induced adverse reaction.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chakkera HA, Kudva Y, Kaplan B. Calcineurin inhibitors: pharmacologic mechanisms impacting both insulin resistance and insulin secretion leading to glucose dysregulation and diabetes mellitus. Clin Pharmacol Ther. 2017;101:114–20.
Ling Q, Huang H, Han Y, Zhang C, Zhang X, Chen K, et al. The tacrolimus-induced glucose homeostasis imbalance in terms of the liver: From bench to bedside. Am J Transpl. 2020;20:701–13.
Rodriguez-Peralvarez M, Colmenero J, Gonzalez A, Gastaca M, Curell A, Caballero-Marcos A, et al. Cumulative exposure to tacrolimus and incidence of cancer after liver transplantation. Am J Transpl. 2022;22:1671–82.
Mizuta K, Kobayashi E, Uchida H, Fujimura A, Kawarasaki H, Hashizume K. Influence of tacrolimus on bile acid and lipid composition in continuously drained bile using a rat model. Comparative study with cyclosporine. Transpl Int. 1999;12:316–22.
Cofan F, Cofan M, Campos B, Guerra R, Campistol JM, Oppenheimer F. Effect of calcineurin inhibitors on low-density lipoprotein oxidation. Transpl Proc. 2005;37:3791–3.
Tory R, Sachs-Barrable K, Goshko CB, Hill JS, Wasan KM. Tacrolimus-induced elevation in plasma triglyceride concentrations after administration to renal transplant patients is partially due to a decrease in lipoprotein lipase activity and plasma concentrations. Transplantation. 2009;88:62–8.
Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, et al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes Rev. 2020;21:e13024.
Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–84.
Asif S, Kim RY, Fatica T, Sim J, Zhao X, Oh Y, et al. Hmgcs2-mediated ketogenesis modulates high-fat diet-induced hepatosteatosis. Mol Metab. 2022;61:101494.
Arima Y, Nakagawa Y, Takeo T, Ishida T, Yamada T, Hino S, et al. Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation. Nat Metab. 2021;3:196–210.
Softic S, Meyer JG, Wang GX, Gupta MK, Batista TM, Lauritzen H, et al. Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab. 2019;30:735–53.e4.
Love S, Mudasir MA, Bhardwaj SC, Singh G, Tasduq SA. Long-term administration of tacrolimus and everolimus prevents high cholesterol-high fructose-induced steatosis in C57BL/6J mice by inhibiting de-novo lipogenesis. Oncotarget. 2017;8:113403–17.
Li S, Zhou H, Xie M, Zhang Z, Gou J, Yang J, et al. Regenerating islet-derived protein 3 gamma (Reg3g) ameliorates tacrolimus-induced pancreatic beta-cell dysfunction in mice by restoring mitochondrial function. Br J Pharmacol. 2022;179:3078–95.
Sikma MA, van Maarseveen EM, van de Graaf EA, Kirkels JH, Verhaar MC, Donker DW, et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transpl. 2015;15:2301–13.
Schaap MM, Zwart EP, Wackers PF, Huijskens I, van de Water B, Breit TM, et al. Dissecting modes of action of non-genotoxic carcinogens in primary mouse hepatocytes. Arch Toxicol. 2012;86:1717–27.
Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017;8:1–8.
d’Avignon DA, Puchalska P, Ercal B, Chang Y, Martin SE, Graham MJ, et al. Hepatic ketogenic insufficiency reprograms hepatic glycogen metabolism and the lipidome. JCI Insight. 2018;3:e99762.
Dieterich IA, Lawton AJ, Peng Y, Yu Q, Rhoads TW, Overmyer KA, et al. Acetyl-CoA flux regulates the proteome and acetyl-proteome to maintain intracellular metabolic crosstalk. Nat Commun. 2019;10:3929.
Rescigno T, Capasso A, Tecce MF. Involvement of nutrients and nutritional mediators in mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase gene expression. J Cell Physiol. 2018;233:3306–14.
Yu J, Shi Y, Zhao K, Yang G, Yu L, Li Y, et al. Enhanced expression of beta cell Ca(V)3.1 channels impairs insulin release and glucose homeostasis. Proc Natl Acad Sci USA. 2020;117:448–53.
Tremblay ML, Giguere V. Phosphatases at the heart of FoxO metabolic control. Cell Metab. 2008;7:101–3.
Smedlund KB, Sanchez ER, Hinds TD Jr. FKBP51 and the molecular chaperoning of metabolism. Trends Endocrinol Metab. 2021;32:862–74.
Zhang C, Chen K, Wei R, Fan G, Cai X, Xu L, et al. The circFASN/miR-33a pathway participates in tacrolimus-induced dysregulation of hepatic triglyceride homeostasis. Signal Transduct Target Ther. 2020;5:23.
Prip-Buus C, Bouthillier-Voisin AC, Kohl C, Demaugre F, Girard J, Pegorier JP. Evidence for an impaired long-chain fatty acid oxidation and ketogenesis in Fao hepatoma cells. Eur J Biochem. 1992;209:291–8.
Kolic J, Beet L, Overby P, Cen HH, Panzhinskiy E, Ure DR, et al. Differential effects of voclosporin and tacrolimus on insulin secretion from human islets. Endocrinology. 2020;161:bqaa162.
Zhang Z, Liu L, Tang H, Jiao W, Zeng S, Xu Y, et al. Immunosuppressive effect of the gut microbiome altered by high-dose tacrolimus in mice. Am J Transpl. 2018;18:1646–56.
Jiao W, Zhang Z, Xu Y, Gong L, Zhang W, Tang H, et al. Butyric acid normalizes hyperglycemia caused by the tacrolimus-induced gut microbiota. Am J Transpl. 2020;20:2413–24.
Balsevich G, Hausl AS, Meyer CW, Karamihalev S, Feng X, Pohlmann ML, et al. Stress-responsive FKBP51 regulates AKT2-AS160 signaling and metabolic function. Nat Commun. 2017;8:1725.
Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–66.
Vila-Brau A, De Sousa-Coelho AL, Mayordomo C, Haro D, Marrero PF. Human HMGCS2 regulates mitochondrial fatty acid oxidation and FGF21 expression in HepG2 cell line. J Biol Chem. 2011;286:20423–30.
Meertens LM, Miyata KS, Cechetto JD, Rachubinski RA, Capone JP. A mitochondrial ketogenic enzyme regulates its gene expression by association with the nuclear hormone receptor PPARalpha. EMBO J. 1998;17:6972–8.
Kostiuk MA, Keller BO, Berthiaume LG. Palmitoylation of ketogenic enzyme HMGCS2 enhances its interaction with PPARalpha and transcription at the Hmgcs2 PPRE. FASEB J. 2010;24:1914–24.
Wickramasinghe NM, Sachs D, Shewale B, Gonzalez DM, Dhanan-Krishnan P, Torre D, et al. PPARdelta activation induces metabolic and contractile maturation of human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2022;29:559–76.e7.
Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–4.
Baeza J, Smallegan MJ, Denu JM. Mechanisms and dynamics of protein acetylation in mitochondria. Trends Biochem Sci. 2016;41:231–44.
Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433:505–14.
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–27.
Helsley RN, Park SH, Vekaria HJ, Sullivan PG, Conroy LR, Sun RC, et al. Ketohexokinase-C regulates global protein acetylation to decrease carnitine palmitoyltransferase 1a-mediated fatty acid oxidation. J Hepatol. 2023;79:25–42.
Wagner GR, Payne RM. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. 2013;288:29036–45.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (No. 82071749, 81703630). The experiments and data analysis were performed in part in the Medical Branch of the Analysis and Test Center of Huazhong University of Science and Technology.
Author information
Authors and Affiliations
Contributions
SLL designed the study, conducted the experiments, analyzed data, and wrote the manuscript. HZ contributed to the design of the study, conducting experiments, and data collection. JL, JY, HMY, MHW, and KSY conducted the experiments. LJ offered technical support. MX supervised the whole project and revised the manuscript. All authors finally approved the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Sl., Zhou, H., Liu, J. et al. Restoration of HMGCS2-mediated ketogenesis alleviates tacrolimus-induced hepatic lipid metabolism disorder. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01300-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01300-0