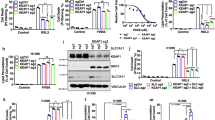
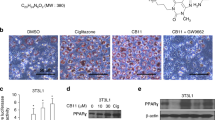
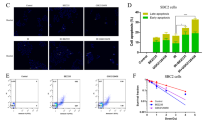
Abstract
Combining radiotherapy with Nrf-2 inhibitor holds promise as a potential therapeutic strategy for radioresistant lung cancer. Here, the radiosensitizing efficacy of a synthetic glucocorticoid clobetasol propionate (CP) in A549 human lung cancer cells was evaluated. CP exhibited potent radiosensitization in lung cancer cells via inhibition of Nrf-2 pathway, leading to elevation of oxidative stress. Transcriptomic studies revealed significant modulation of pathways related to ferroptosis, fatty acid and glutathione metabolism. Consistent with these findings, CP treatment followed by radiation exposure showed characteristic features of ferroptosis in terms of mitochondrial swelling, rupture and loss of cristae. Ferroptosis is a form of regulated cell death triggered by iron-dependent ROS accumulation and lipid peroxidation. In combination with radiation, CP showed enhanced iron release, mitochondrial ROS, and lipid peroxidation, indicating ferroptosis induction. Further, iron chelation, inhibition of lipid peroxidation or scavenging mitochondrial ROS prevented CP-mediated radiosensitization. Nrf-2 negatively regulates ferroptosis through upregulation of antioxidant defense and iron homeostasis. Interestingly, Nrf-2 overexpressing A549 cells were refractory to CP-mediated ferroptosis induction and radiosensitization. Thus, this study identified anti-psoriatic drug clobetasol propionate can be repurposed as a promising radiosensitizer for Keap-1 mutant lung cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data will be made available upon reasonable request.
References
Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol. 2021;25:45–52.
Uzel EK, Figen M, Uzel Ö. Radiotherapy in lung cancer: current and future role. Sisli Etfal Hastan Tip Bul. 2019;53:353–60.
Aravindan N, Aravindan S, Pandian V, Khan FH, Ramraj SK, Natt P, et al. Acquired tumor cell radiation resistance at the treatment site is mediated through radiation-orchestrated intercellular communication. Int J Radiat Oncol Biol Phys. 2014;88:677–85.
Césaire M, Montanari J, Curcio H, Lerouge D, Gervais R, Demontrond P, et al. Radioresistance of non-small cell lung cancers and therapeutic perspectives. Cancers. 2022;14:2829.
Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B. Molecular mechanisms of chemo‐and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021;2:315–40.
Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:1–11.
Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol. 2004;22:2214–32.
Choi E, Jung B, Lee S, Yoo H, Shin E, Ko H, et al. A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene. 2017;36:5285–95.
Tian Y, Liu Q, He X, Yuan X, Chen Y, Chu Q, et al. Emerging roles of Nrf2 signal in non-small cell lung cancer. J Hematol Oncol. 2016;9:1–9.
Sánchez-Ortega M, Carrera AC, Garrido A. Role of NRF2 in lung cancer. Cells. 2021;10:1879.
Singh A, Bodas M, Wakabayashi N, Bunz F, Biswal S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid Redox Signal. 2010;13:1627–37.
Xiang Y, Ye W, Huang C, Yu D, Chen H, Deng T, et al. Brusatol enhances the chemotherapy efficacy of gemcitabine in pancreatic cancer via the Nrf2 signalling pathway. Oxid Med Cell Longev. 2018;2018:2360427.
Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA. 2011;108:1433–8.
Ji L, Moghal N, Zou X, Fang Y, Hu S, Wang Y, et al. The NRF2 antagonist ML385 inhibits PI3K‐mTOR signaling and growth of lung squamous cell carcinoma cells. Cancer Med. 2023;12:5688–702.
Qin S, He X, Lin H, Schulte BA, Zhao M, Tew KD, et al. Nrf2 inhibition sensitizes breast cancer stem cells to ionizing radiation via suppressing DNA repair. Free Radic Biol Med. 2021;169:238–47.
Sun X, Dong M, Gao Y, Wang Y, Du L, Liu Y, et al. Metformin increases the radiosensitivity of non-small cell lung cancer cells by destabilizing NRF2. Biochem Pharmacol. 2022;199:114981.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30:1704007.
Stockwell BR. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–21.
Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85.
Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–90.
Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–62.
Su J, Bian C, Zheng Z, Wang H, Meng L, Xin Y, et al. Cooperation effects of radiation and ferroptosis on tumor suppression and radiation injury. Front Cell Dev Biol. 2022;10:951116.
Ye LF, Chaudhary KR, Zandkarimi F, Harken AD, Kinslow CJ, Upadhyayula PS, et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem Biol. 2020;15:469–84.
Li J, Cao F, Yin H-l, Huang Z-j, Lin Z-t, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88.
Anandhan A, Dodson M, Shakya A, Chen J, Liu P, Wei Y, et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci Adv. 2023;9:eade9585.
Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107.
Shakya A, McKee NW, Dodson M, Chapman E, Zhang DD. Anti-ferroptotic effects of Nrf2: Beyond the antioxidant response. Mol Cells. 2023;46:165.
Dodson M, Anandhan A, Zhang DD. MGST1, a new soldier of NRF2 in the battle against ferroptotic death. Cell Chem Biol. 2021;28:741–2.
Maurya DK. ColonyCountJ: a user-friendly image J Add-on program for quantification of different colony parameters in clonogenic assay. J Clin Toxicol. 2017;7:1–4.
Chang DS, Lasley FD, Das IJ, Mendonca MS, Dynlacht JR. Radiation survival models, SLD, PLD, and dose rate. In: Chang DS, Lasley FD, Das IJ, Mendonca MS, Dynlacht JR, Editors. Basic Radiotherapy Physics and Biology. Springer International Publishing, Cham; 2021. p 243–53.
Patwardhan R, Sharma D, Checker R, Sandur SK. Mitigation of radiation-induced hematopoietic injury via regulation of cellular MAPK/phosphatase levels and increasing hematopoietic stem cells. Free Radic Biol Med. 2014;68:52–64.
Jayakumar S, Kunwar A, Sandur SK, Pandey BN, Chaubey RC. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim Biophys Acta Gen Subj. 2014;1840:485–94.
Pachpatil PK, Kanojia SV, Ghosh A, Majumdar AG, Wadawale A, Mohapatra M, et al. Stable, triplet ground state BODIPY-TEMPO diradical as a selective turn on fluorescence sensor for intracellular labile iron pool. Sens Actuators B Chem. 2022;370:132474.
Sharma D, Eichelberg MR, Haag JD, Meilahn AL, Muelbl MJ, Schell K, et al. Effective flow cytometric phenotyping of cells using minimal amounts of antibody. Biotechniques. 2012;53:57–60.
Andrews S, Krueger F, Seconds-Pichon A, Biggins F, Wingett SF. A quality control tool for high throughput sequence data. Babraham Bioinforma Babraham Inst. 2014;1:1.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890.
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–9.
Soneson C, Love MI, Robinson MD. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:1–21.
Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–9.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–7.
Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43:W566–70.
Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay methodsynergy quantification method. Cancer Res. 2010;70:440–6.
Bloom DA, Jaiswal AK. Phosphorylation of Nrf2 at Ser40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2, but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD (P) H: quinone oxidoreductase-1 gene expression. J Biol Chem. 2003;278:44675–82.
Jayakumar S, Pal D, Sandur SK. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res. 2015;779:33–45.
Dixon SJ, Stockwell BR. The hallmarks of ferroptosis. Annu Rev Cancer Biol. 2019;3:35–54.
Battaglia AM, Chirillo R, Aversa I, Sacco A, Costanzo F, Biamonte F. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020;9:1505.
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, et al. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420.
Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173:321–37.e10.
Xia D, Zhang XR, Ma YL, Zhao ZJ, Zhao R, Wang YY. Nrf2 promotes esophageal squamous cell carcinoma (ESCC) resistance to radiotherapy through the CaMKIIα-associated activation of autophagy. Cell Biosci. 2020;10:1–12.
Zhao Q, Mao A, Yan J, Sun C, Di C, Zhou X, et al. Downregulation of Nrf2 promotes radiation-induced apoptosis through Nrf2 mediated Notch signaling in non-small cell lung cancer cells. Int J Oncol. 2016;48:765–73.
Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, et al. Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth the loss of Keap1 activity in prostate cancer cells. Mol Cancer Ther. 2010;9:336–46.
Feng L, Zhao K, Sun L, Yin X, Zhang J, Liu C, et al. SLC7A11 regulated by NRF2 modulates esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis. J Transl Med. 2021;19:1–16.
Liu X, Sun C, Liu B, Jin X, Li P, Zheng X, et al. Genistein mediates the selective radiosensitizing effect in NSCLC A549 cells via inhibiting methylation of the keap1 gene promoter region. Oncotarget. 2016;7:27267.
Sun M, Pan D, Chen Y, Li Y, Gao K, Hu B. Coroglaucigenin enhances the radiosensitivity of human lung cancer cells through Nrf2/ROS pathway. Oncotarget. 2017;8:32807.
Xu S, Huang H, Tang D, Xing M, Zhao Q, Li J, et al. Diallyl disulfide attenuates ionizing radiation-induced migration and invasion by suppressing Nrf2 signaling in non–small-cell lung cancer. Dose-Response. 2021;19:15593258211033114.
Petrini JH, Stracker TH. The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol. 2003;13:458–62.
Schultz LB, Chehab NH, Malikzay A, Halazonetis TD. p53 binding protein 1 (53BP1) is an early participant in the cellular response to DNA double-strand breaks. J Cell Biol. 2000;151:1381–90.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82.
Feng H, Stockwell BR. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018;16:e2006203.
Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–76.
Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent-and AIF-mediated cell death. Cell Metab. 2008;8:237–48.
Liu CY, Liu CC, Li AFY, Hsu TW, Lin JH, Hung SC, et al. Glutathione peroxidase 4 expression predicts poor overall survival in patients with resected lung adenocarcinoma. Sci Rep. 2022;12:20462.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
McDonald JT, Kim K, Norris AJ, Vlashi E, Phillips TM, Lagadec C, et al. Ionizing radiation activates the Nrf2 antioxidant response. Cancer Res. 2010;70:8886–95.
Kiesslich T, Mayr C, Neureiter D. NRF2: The key to tumor-and patient-dependent chemosensitivity in biliary tract cancer? EBioMedicine. 2019;49:9–10.
Du Y, Guo Z. Recent progress in ferroptosis: inducers and inhibitors. Cell Death Discov. 2022;8:501.
Lu Z, Xiao B, Chen W, Tang T, Chen X. The potential of ferroptosis combined with radiotherapy in cancer treatment. Front Oncol. 2023;13:158.
Acknowledgements
Authors acknowledge the scientific inputs provided by Dr. Deepak Sharma, SO/G, RB&HSD, Dr. Dharmendra K. Maurya, SO/G, RB&HSD and Dr.Dibakar Goswami, SO/G, BOD. Authors also acknowledge the technical help provided by Ms. Binita Kislay Kumar for flow cytometry and Mr. B. A. Naidu & Mr. Deepak Kathole for help during irradiation of cells. Authors would like to thank ACTREC’s TEM facility and animal house facility. The authors acknowledge the funding provided by DAE, Government of India to conduct research as outlined in this article.
Author information
Authors and Affiliations
Contributions
Conceptualization: SKS; Methodology: AR, PP, SJ, GCP; Investigation: AR, SJ, SP, DD, GCP; Visualization: AR, RSP, SJ, SP, DD; Funding acquisition: SKS; Resources: SKS, SJ, VG; Project administration: SKS; Supervision: SKS; Writing – original draft: AR; Writing – review & editing: RSP, SJ, SKS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Study does not include any data with human identification information. No studies on human participants were performed.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rai, A., Patwardhan, R.S., Jayakumar, S. et al. Clobetasol propionate, a Nrf-2 inhibitor, sensitizes human lung cancer cells to radiation-induced killing via mitochondrial ROS-dependent ferroptosis. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01233-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01233-8