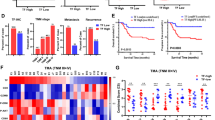
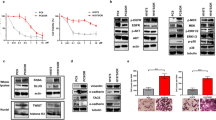
Abstract
Although ALK tyrosine kinase inhibitors (ALK-TKIs) have shown remarkable benefits in EML4-ALK positive NSCLC patients compared to conventional chemotherapy, the optimal sequence of ALK-TKIs treatment remains unclear due to the emergence of primary and acquired resistance and the lack of potential prognostic biomarkers. In this study, we systematically explored the validity of sequential ALK inhibitors (alectinib, lorlatinib, crizotinib, ceritinib and brigatinib) for a heavy-treated patient with EML4-ALK fusion via developing an in vitro and in vivo drug testing system based on patient-derived models. Based on the patient-derived models and clinical responses of the patient, we found that crizotinib might inhibit proliferation of EML4-ALK positive tumors resistant to alectinib and lorlatinib. In addition, NSCLC patients harboring the G1269A mutation, which was identified in alectinib, lorlatinib and crizotinib-resistant NSCLC, showed responsiveness to brigatinib and ceritinib. Transcriptomic analysis revealed that brigatinib suppressed the activation of multiple inflammatory signaling pathways, potentially contributing to its anti-tumor activity. Moreover, we constructed a prognostic model based on the expression of IL6, CXCL1, and CXCL5, providing novel perspectives for predicting prognosis in EML4-ALK positive NSCLC patients. In summary, our results delineate clinical responses of sequential ALK-TKIs treatments and provide insights into the mechanisms underlying the superior effects of brigatinib in patients harboring ALKG1269A mutation and resistant towards alectinib, lorlatinib and crizotinib. The molecular signatures model based on the combination of IL6, CXCL1 and CXCL5 has the potential to predict prognosis of EML4-ALK positive NSCLC patients.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Qin Z, Sun HH, Yue MT, Pan XW, Chen L, Feng XH, et al. Phase separation of EML4-ALK in firing downstream signaling and promoting lung tumorigenesis. Cell Discov. 2021;7:33.
Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53.
Camidge DR, Dziadziuszko R, Peters S, Mok T, Noe J, Nowicka M, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14:1233–43.
Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39.
Lin JJ, Zhu VW, Schoenfeld AJ, Yeap BY, Saxena A, Ferris LA, et al. Brigatinib in patients with alectinib-refractory ALK-positive NSCLC. J Thorac Oncol. 2018;13:1530–8.
Bearz A, De Carlo E, Del Conte A, Spina M, Da Ros V, Bertoli E, et al. The change in paradigm for NSCLC patients with EML4-ALK translocation. Int J Mol Sci. 2022;23:7322.
Yoda S, Lin JJ, Lawrence MS, Burke BJ, Friboulet L, Langenbucher A, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov. 2018;8:714–29.
Cho BC, Obermannova R, Bearz A, McKeage M, Kim DW, Batra U, et al. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)-positive NSCLC: primary efficacy results from the ASCEND-8 study. J Thorac Oncol. 2019;14:1255–65.
Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020;31:1056–64.
Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–29.
Horn L, Wang Z, Wu G, Poddubskaya E, Mok T, Reck M, et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7:1617–25.
Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16:2091–108.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38.
Soria JC, Tan DSW, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–29.
Lin JJ, Zhu VW, Yoda S, Yeap BY, Schrock AB, Dagogo-Jack I, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36:1199–206.
Zhang S, Anjum R, Squillace R, Nadworny S, Zhou T, Keats J, et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin Cancer Res. 2016;22:5527–38.
Qian MJ, Yan FJ, Wang WW, Du JM, Yuan T, Wu RL, et al. Deubiquitinase JOSD2 stabilizes YAP/TAZ to promote cholangiocarcinoma progression. Acta Pharm Sin B. 2021;11:4008–19.
Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, et al. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumour-associated Treg cells. Nat Commun. 2017;8:14649.
Saito K, Orimo K, Kubo T, Tamari M, Yamada A, Motomura K, et al. Laundry detergents and surfactants-induced eosinophilic airway inflammation by increasing IL-33 expression and activating ILC2s. Allergy. 2023;78:1878–92.
Finetti F, Paradisi L, Bernardi C, Pannini M, Trabalzini L. Cooperation between prostaglandin E2 and epidermal growth factor receptor in cancer progression: a dual target for cancer therapy. Cancers. 2023;15:2374.
Kiełbowski K, Żychowska J, Becht R. Anaplastic lymphoma kinase inhibitors-a review of anticancer properties, clinical efficacy, and resistance mechanisms. Front Pharmacol. 2023;14:1285374.
Zhao W, Yu D, Zhai Y, Sun SY. ALK inhibitors downregulate the expression of death receptor 4 in ALK-mutant lung cancer cells via facilitating Fra-1 and c-Jun degradation and subsequent AP-1 suppression. Neoplasia. 2023;42:100908.
Kwok HH, Li HY, Yang JS, Deng JY, Lee NC, Au TW, et al. Single-cell transcriptomic analysis uncovers intratumoral heterogeneity and drug-tolerant persister in ALK-rearranged lung adenocarcinoma. Cancer Commun. 2023;43:951–5.
Zhang XC, Wang J, Shao GG, Wang Q, Qu X, Wang B, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun. 2019;10:1772.
Song XL, Zhong H, Qu XJ, Yang LS, Jiang B. Two novel strategies to overcome the resistance to ALK tyrosine kinase inhibitor drugs: Macrocyclic inhibitors and proteolysis-targeting chimeras. MedComm. 2021;2:341–50.
Shi YK, Fang J, Hao XZ, Zhang SC, Liu YP, Wang L, et al. Safety and activity of WX-0593 (Iruplinalkib) in patients with ALK- or ROS1-rearranged advanced non-small cell lung cancer: a phase 1 dose-escalation and dose-expansion trial. Signal Transduct Target Ther. 2022;7:25.
Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27:iii42–iii50.
Liu J, Cui SH, Pan F, Ni YQ, Zhong H, Xiong LW, et al. Feasibility of continuing crizotinib therapy after RECIST-PD in advanced non-small cell lung cancer patients with ALK/ROS-1 mutations. J Cancer. 2018;9:1863–9.
Dagogo-Jack I, Rooney M, Lin JJ, Nagy RJ, Yeap BY, Hubbeling H, et al. Treatment with next-generation ALK inhibitors fuels plasma ALK mutation diversity. Clin Cancer Res. 2019;25:6662–70.
Recondo G, Mezquita L, Facchinetti F, Planchard D, Gazzah A, Bigot L, et al. Diverse resistance mechanisms to the third-generation ALK inhibitor lorlatinib in ALK-rearranged lung cancer. Clin Cancer Res. 2020;26:242–55.
Xia B, Nagasaka M, Zhu VW, Ou SI, Soo RA. How to select the best upfront therapy for metastatic disease? Focus on ALK-rearranged non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2020;9:2521–34.
Wang L, Wang W. Safety and efficacy of anaplastic lymphoma kinase tyrosine kinase inhibitors in non‑small cell lung cancer (Review). Oncol Rep. 2021;45:13–28.
Carvalho S, Troost EG, Bons J, Menheere P, Lambin P, Oberije C. Prognostic value of blood-biomarkers related to hypoxia, inflammation, immune response and tumour load in non-small cell lung cancer - a survival model with external validation. Radiother Oncol. 2016;119:487–94.
Dehing-Oberije C, Aerts H, Yu S, De Ruysscher D, Menheere P, Hilvo M, et al. Development and validation of a prognostic model using blood biomarker information for prediction of survival of non-small-cell lung cancer patients treated with combined chemotherapy and radiation or radiotherapy alone (NCT00181519, NCT00573040, and NCT00572325). Int J Radiat Oncol Biol Phys. 2011;81:360–8.
Yu KH, Zhang C, Berry GJ, Altman RB, Ré C, Rubin DL, et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun. 2016;7:12474.
Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–89.
Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–7.
Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–78.
Yang Y, Ding LL, Hu Q, Xia J, Sun JJ, Wang XD, et al. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16:141.
Gong YZ, Ma H, Ruan GT, Zhu LC, Liao XW, Wang S, et al. Diagnosis and prognostic value of C-X-C motif chemokine ligand 1 in colon adenocarcinoma based on The Cancer Genome Atlas and Guangxi cohort. J Cancer. 2021;12:5506–18.
Jia SN, Han YB, Yang R, Yang ZC. Chemokines in colon cancer progression. Semin Cancer Biol. 2022;86:400–7.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U21A20420) and the Science and Technology Development Project of Hangzhou (202204A08). We thank Dr. Jian-ming Zeng (University of Macau) and all the members of his bioinformatics team, biotrainee, for generously sharing their experience and codes. The use of the biorstudio high-performance computing cluster (http://biotrainee.vip:9903/) at Biotrainee and the Shanghai HS Biotech Co., Ltd for conducting the research reported in this paper.
Author information
Authors and Affiliations
Contributions
FJG, XYD, and YQ were responsible for experiment design, biological information analysis, and manuscript writing. XNL and CMZ was responsible for results description, handling of pictures and tables. XYX was responsible for clinical samples required for research. YDC and RHG assisted animal modeling and revised the manuscript. HZ and QJH were responsible for language embellishment and manuscript writing. SLM, XQC, and BY were responsible for the research design, supervising the study, and editing manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, Fj., Dai, Xy., Qiu, Y. et al. Inflammation-related molecular signatures involved in the anticancer activities of brigatinib as well as the prognosis of EML4-ALK lung adenocarcinoma patient. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01230-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01230-x