Abstract
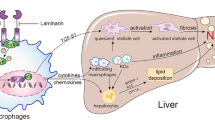
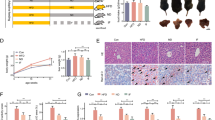
Non-alcoholic fatty liver disease (NAFLD) is a common metabolic disease that is substantially associated with obesity-induced chronic inflammation. Macrophage activation and macrophage-medicated inflammation play crucial roles in the development and progression of NAFLD. Furthermore, fibroblast growth factor receptor 1 (FGFR1) has been shown to be essentially involved in macrophage activation. This study investigated the role of FGFR1 in the NAFLD pathogenesis and indicated that a high-fat diet (HFD) increased p-FGFR1 levels in the mouse liver, which is associated with increased macrophage infiltration. In addition, macrophage-specific FGFR1 knockout or administration of FGFR1 inhibitor markedly protected the liver from HFD-induced lipid accumulation, fibrosis, and inflammatory responses. The mechanistic study showed that macrophage-specific FGFR1 knockout alleviated HFD-induced liver inflammation by suppressing the activation of MAPKs and TNF signaling pathways and reduced fat deposition in hepatocytes, thereby inhibiting the activation of hepatic stellate cells. In conclusion, the results of this research revealed that FGFR1 could protect the liver of HFD-fed mice by inhibiting MAPKs/TNF-mediated inflammatory responses in macrophages. Therefore, FGFR1 can be employed as a target to prevent the development and progression of NAFLD.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017;27:R1147–R51.
Pineiro-Carrero VM, Pineiro EO. Liver. Pediatrics. 2004;113:1097–106.
Younossi ZM. Non-alcoholic fatty liver disease—a global public health perspective. J Hepatol. 2019;70:531–44.
Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24.
Barreby E, Chen P, Aouadi M. Macrophage functional diversity in NAFLD—more than inflammation. Nat Rev Endocrinol. 2022;18:461–72.
Gordon S. The macrophage. Bioessays. 1995;17:977–86.
Alisi A, Carpino G, Oliveira FL, Panera N, Nobili V, Gaudio E. The role of tissue macrophage-mediated inflammation on NAFLD pathogenesis and its clinical implications. Mediators Inflamm. 2017;2017:8162421.
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–49.
Wang S, Cao S, Arhatte M, Li D, Shi Y, Kurz S, et al. Adipocyte Piezo1 mediates obesogenic adipogenesis through the FGF1/FGFR1 signaling pathway in mice. Nat Commun. 2020;11:2303.
Choi Y, Jang S, Choi MS, Ryoo ZY, Park T. Increased expression of FGF1-mediated signaling molecules in adipose tissue of obese mice. J Physiol Biochem. 2016;72:157–67.
Wang C, Li Y, Li H, Zhang Y, Ying Z, Wang X, et al. Disruption of FGF signaling ameliorates inflammatory response in hepatic stellate cells. Front Cell Dev Biol. 2020;8:601.
Arangalage D, Degrauwe N, Michielin O, Monney P, Ozdemir BC. Pathophysiology, diagnosis and management of cardiac toxicity induced by immune checkpoint inhibitors and BRAF and MEK inhibitors. Cancer Treat Rev. 2021;100:102282.
Diecke S, Quiroga-Negreira A, Redmer T, Besser D. FGF2 signaling in mouse embryonic fibroblasts is crucial for self-renewal of embryonic stem cells. Cells Tissues Organs. 2008;188:52–61.
Li D, Xia L, Huang P, Wang Z, Guo Q, Huang C, et al. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discov. 2023;9:17.
Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, et al. AZD4547: an orally bioavailable, potent, and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–56.
Pan Y, Wang Y, Cai L, Cai Y, Hu J, Yu C, et al. Inhibition of high glucose-induced inflammatory response and macrophage infiltration by a novel curcumin derivative prevents renal injury in diabetic rats. Br J Pharmacol. 2012;166:1169–82.
Charni-Natan M, Goldstein I. Protocol for primary mouse hepatocyte isolation. STAR Protoc. 2020;1:100086.
Raj T, Kanellakis P, Pomilio G, Jennings G, Bobik A, Agrotis A. Inhibition of fibroblast growth factor receptor signaling attenuates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1845–51.
Liu X, Mashour GA, Webster HF, Kurtz A. Basic FGF and FGF receptor 1 are expressed in microglia during experimental autoimmune encephalomyelitis: temporally distinct expression of midkine and pleiotrophin. Glia. 1998;24:390–7.
Pinto AR, Godwin JW, Chandran A, Hersey L, Ilinykh A, Debuque R, et al. Age-related changes in tissue macrophages precede cardiac functional impairment. Aging (Albany NY). 2014;6:399–413.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Kazankov K, Jorgensen SMD, Thomsen KL, Moller HJ, Vilstrup H, George J, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. 2019;16:145–59.
Xu L, Liu W, Bai F, Xu Y, Liang X, Ma C, et al. Hepatic macrophage as a key player in fatty liver disease. Front Immunol. 2021;12:708978.
Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102.
Webster JD, Vucic D. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front Cell Dev Biol. 2020;8:365.
Zhang Y, Wu B, Zhang H, Ge X, Ying S, Hu M, et al. Inhibition of MD2-dependent inflammation attenuates the progression of non-alcoholic fatty liver disease. J Cell Mol Med. 2018;22:936–47.
Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61:1294–303.
Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–E40.
Gao J, Wei B, de Assuncao TM, Liu Z, Hu X, Ibrahim S, et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144–54.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–66.
Dewidar B, Meyer C, Dooley S, Meindl-Beinker AN. TGF-beta in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells. 2019;8:1419.
Cheng Q, Li C, Yang CF, Zhong YJ, Wu D, Shi L, et al. Methyl ferulic acid attenuates liver fibrosis and hepatic stellate cell activation through the TGF-beta1/Smad and NOX4/ROS pathways. Chem Biol Interact. 2019;299:131–9.
Lee YS, Wollam J, Olefsky JM. An integrated view of immunometabolism. Cell. 2018;172:22–40.
Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and beta-cell abnormalities. Nat Rev Endocrinol. 2020;16:81–90.
Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–42.
Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–74.
Yang B, Luo W, Wang M, Tang Y, Zhu W, Jin L, et al. Macrophage-specific MyD88 deletion and pharmacological inhibition prevents liver damage in non-alcoholic fatty liver disease via reducing inflammatory response. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166480.
Wang Y, Qian Y, Fang Q, Zhong P, Li W, Wang L, et al. Author Correction: Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat Commun. 2018;9:16185.
Lawan A, Bennett AM. Mitogen-activated protein kinase regulation in hepatic metabolism. Trends Endocrinol Metab. 2017;28:868–78.
Wu L, Sun J, Liu L, Du X, Liu Y, Yan X, et al. Anti-toll-like receptor 2 antibody ameliorates hepatic injury, inflammation, fibrosis and steatosis in obesity-related metabolic disorder rats via regulating MAPK and NF-kappaB pathways. Int Immunopharmacol. 2020;82:106368.
Jiang J, Yan L, Shi Z, Wang L, Shan L, Efferth T. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus Changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-kappaB and MAPKs. Phytomedicine. 2019;64:153082.
Zhao W, Yan Y, Xiao Z, Wang M, Xu M, Wang Z, et al. Bicyclol ameliorates nonalcoholic fatty liver disease in mice via inhibiting MAPKs and NF-kappaB signaling pathways. Biomed Pharmacother. 2021;141:111874.
Wu L, Wang Y, Chi G, Shen B, Tian Y, Li Z, et al. Morin reduces inflammatory responses and alleviates lipid accumulation in hepatocytes. J Cell Physiol. 2019;234:19785–98.
Huang Y, Wang F, Li H, Xu S, Xu W, Pan X, et al. Inhibition of fibroblast growth factor receptor by AZD4547 protects against inflammation in septic mice. Inflammation. 2019;42:1957–67.
Chen X, Zhang X, Xu J, Zhao Y, Bao J, Zheng Z, et al. AZD4547 attenuates lipopolysaccharide-induced acute kidney injury by inhibiting inflammation: the role of FGFR1 in renal tubular epithelial cells. Drug Des Devel Ther. 2020;14:833–44.
Xu Z, Luo W, Chen L, Zhuang Z, Yang D, Qian J, et al. Ang II (Angiotensin II)-induced FGFR1 (Fibroblast Growth Factor Receptor 1) activation in tubular epithelial cells promotes hypertensive kidney fibrosis and injury. Hypertension. 2022;79:2028–41.
Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–66.
Acknowledgements
Financial support was provided by the Medical and Health Science Research Project of Zhejiang Province (2023XY164 to LJH, 2022KY348 to WZ), Key Research Project of Wenzhou City (ZY2021021 to YW).
Author information
Authors and Affiliations
Contributions
YNZ, ZDL, TY, and YW contributed to the literature search and study design. YNZ, TXX, TYJ, YSJ, WZ, KYL, and YW performed the experiments and analyzed the data. LJH and KYL provided technical help. TXX, TYJ, LJH, and YW participated in the drafting of the article. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Yn., Liu, Zd., Yan, T. et al. Macrophage-specific FGFR1 deletion alleviates high-fat-diet-induced liver inflammation by inhibiting the MAPKs/TNF pathways. Acta Pharmacol Sin 45, 988–1001 (2024). https://doi.org/10.1038/s41401-024-01226-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-024-01226-7