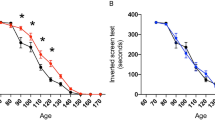
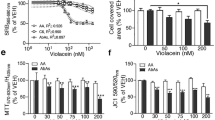
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease with progressive loss of motor neurons in the spinal cord, cerebral cortex and brain stem. ALS is characterized by gradual muscle atrophy and dyskinesia. The limited knowledge on the pathology of ALS has impeded the development of therapeutics for the disease. Previous studies have shown that autophagy and astrocyte-mediated neuroinflammation are involved in the pathogenesis of ALS, while 5HTR2A participates in the early stage of astrocyte activation, and 5HTR2A antagonism may suppress astrocyte activation. In this study, we evaluated the therapeutic effects of desloratadine (DLT), a selective 5HTR2A antagonist, in human SOD1G93A (hSOD1G93A) ALS model mice, and elucidated the underlying mechanisms. HSOD1G93A mice were administered DLT (20 mg·kg−1·d−1, i.g.) from the age of 8 weeks for 10 weeks or until death. ALS onset time and lifespan were determined using rotarod and righting reflex tests, respectively. We found that astrocyte activation accompanying with serotonin receptor 2 A (5HTR2A) upregulation in the spinal cord was tightly associated with ALS-like pathology, which was effectively attenuated by DLT administration. We showed that DLT administration significantly delayed ALS symptom onset time, prolonged lifespan and ameliorated movement disorders, gastrocnemius injury and spinal motor neuronal loss in hSOD1G93A mice. Spinal cord-specific knockdown of 5HTR2A by intrathecal injection of adeno-associated virus9 (AAV9)-si-5Htr2a also ameliorated ALS pathology in hSOD1G93A mice, and occluded the therapeutic effects of DLT administration. Furthermore, we demonstrated that DLT administration promoted autophagy to reduce mutant hSOD1 levels through 5HTR2A/cAMP/AMPK pathway, suppressed oxidative stress through 5HTR2A/cAMP/AMPK/Nrf2-HO-1/NQO-1 pathway, and inhibited astrocyte neuroinflammation through 5HTR2A/cAMP/AMPK/NF-κB/NLRP3 pathway in the spinal cord of hSOD1G93A mice. In summary, 5HTR2A antagonism shows promise as a therapeutic strategy for ALS, highlighting the potential of DLT in the treatment of the disease.

DLT as a 5HTR2A antagonist effectively promoted autophagy to reduce mutant hSOD1 level through 5HTR2A/cAMP/AMPK pathway, suppressed oxidative stress through 5HTR2A/cAMP/AMPK/Nrf2-HO-1/NQO-1 pathway, and inhibited astrocytic neuroinflammation through 5HTR2A/cAMP/AMPK/NF-κB/NLRP3 pathway in the spinal cord of hSOD1G93A mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Feldman EL, Goutman SA, Petri S, Mazzini L, Savelieff MG, Shaw PJ, et al. Amyotrophic lateral sclerosis. Lancet. 2022;400:1363–80.
Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc. 2018;93:1617–28.
Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–28.
Cetin H, Rath J, Fuzi J, Reichardt B, Fulop G, Koppi S, et al. Epidemiology of amyotrophic lateral sclerosis and effect of riluzole on disease course. Neuroepidemiology. 2015;44:6–15.
Sawada H. Clinical efficacy of edaravone for the treatment of amyotrophic lateral sclerosis. Expert Opin Pharmacother. 2017;18:735–8.
Petrov D, Mansfield C, Moussy A, Hermine O. ALS clinical trials review: 20 years of failure. are we any closer to registering a new treatment? Front Aging Neurosci. 2017;9:68.
Sau D, De Biasi S, Vitellaro-Zuccarello L, Riso P, Guarnieri S, Porrini M, et al. Mutation of SOD1 in ALS: a gain of a loss of function. Hum Mol Genet. 2007;16:1604–18.
Nishimura AL, Shum C, Scotter EL, Abdelgany A, Sardone V, Wright J, et al. Allele-specific knockdown of ALS-associated mutant TDP-43 in neural stem cells derived from induced pluripotent stem cells. PLoS One. 2014;9:e91269.
Trist BG, Genoud S, Roudeau S, Rookyard A, Abdeen A, Cottam V, et al. Altered SOD1 maturation and post-translational modification in amyotrophic lateral sclerosis spinal cord. Brain. 2022;145:3108–30.
Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, et al. Distinct roles for motor neuron autophagy early and late in the SOD1(G93A) mouse model of ALS. Proc Natl Acad Sci USA. 2017;114:E8294–E303.
Debye B, Schmulling L, Zhou L, Rune G, Beyer C, Johann S. Neurodegeneration and NLRP3 inflammasome expression in the anterior thalamus of SOD1(G93A) ALS mice. Brain Pathol. 2018;28:14–27.
Guo F, Liu X, Cai H, Le W. Autophagy in neurodegenerative diseases: pathogenesis and therapy. Brain Pathol. 2018;28:3–13.
Castillo K, Nassif M, Valenzuela V, Rojas F, Matus S, Mercado G, et al. Trehalose delays the progression of amyotrophic lateral sclerosis by enhancing autophagy in motoneurons. Autophagy. 2013;9:1308–20.
Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10:253–63.
Johann S, Heitzer M, Kanagaratnam M, Goswami A, Rizo T, Weis J, et al. NLRP3 inflammasome is expressed by astrocytes in the SOD1 mouse model of ALS and in human sporadic ALS patients. Glia. 2015;63:2260–73.
Swami M. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Med. 2011;17:1059.
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–73.
Dennys C, Baggio C, Rodrigo R, Roussel F, Kulinich A, Heintzman S, et al. EphA4 targeting agents protect motor neurons from cell death induced by amyotrophic lateral sclerosis -astrocytes. iScience. 2022;25:104877.
Mahesh G, Jaiswal P, Dey S, Sengupta J, Mukherjee S. Cloning, expression, purification and characterization of oligomeric states of the native 5HT2A G-protein-coupled receptor. Protein Pept Lett. 2018;25:390–7.
Thibault K, Van Steenwinckel J, Brisorgueil MJ, Fischer J, Hamon M, Calvino B, et al. Serotonin 5-HT2A receptor involvement and Fos expression at the spinal level in vincristine-induced neuropathy in the rat. Pain. 2008;140:305–22.
Nishiyama T. Effects of a 5-HT2A receptor antagonist, sarpogrelate on thermal or inflammatory pain. Eur J Pharmacol. 2005;516:18–22.
Wu C, Singh SK, Dias P, Kumar S, Mann DM. Activated astrocytes display increased 5-HT2a receptor expression in pathological states. Exp Neurol. 1999;158:529–33.
Fritz I, Wagner P, Broberg P, Einefors R, Olsson H. Desloratadine and loratadine stand out among common H1-antihistamines for association with improved breast cancer survival. Acta Oncol. 2020;59:1103–9.
Lu J, Zhang C, Lv J, Zhu X, Jiang X, Lu W, et al. Antiallergic drug desloratadine as a selective antagonist of 5HT2A receptor ameliorates pathology of Alzheimer’s disease model mice by improving microglial dysfunction. Aging Cell. 2020;20:e13286.
Ionescu A, Gradus T, Altman T, Maimon R, Saraf Avraham N, Geva M, et al. Targeting the Sigma-1 receptor via pridopidine ameliorates central features of ALS pathology in a SOD1(G93A) Model. Cell Death Dis. 2019;10:210.
Tsuburaya N, Homma K, Higuchi T, Balia A, Yamakoshi H, Shibata N, et al. A small-molecule inhibitor of SOD1-Derlin-1 interaction ameliorates pathology in an ALS mouse model. Nat Commun. 2018;9:2668.
Lee JD, Liu N, Levin SC, Ottosson L, Andersson U, Harris HE, et al. Therapeutic blockade of HMGB1 reduces early motor deficits, but not survival in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. J Neuroinflammation. 2019;16:45.
Tan H, Chen M, Pang D, Xia X, Du C, Yang W, et al. LanCL1 promotes motor neuron survival and extends the lifespan of amyotrophic lateral sclerosis mice. Cell Death Differ. 2020;27:1369–82.
Zhu DY, Lu J, Xu R, Yang JZ, Meng XR, Ou-Yang XN, et al. FX5, a non-steroidal glucocorticoid receptor antagonist, ameliorates diabetic cognitive impairment in mice. Acta Pharmacol Sin. 2022;43:2495–510.
Zhou QM, Zhang JJ, Li S, Chen S, Le WD. n-butylidenephthalide treatment prolongs life span and attenuates motor neuron loss in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. CNS Neurosci Ther. 2017;23:375–85.
Zhang X, Chen S, Lu K, Wang F, Deng J, Xu Z, et al. Verapamil ameliorates motor neuron degeneration and improves lifespan in the SOD1(G93A) mouse model of ALS by enhancing autophagic flux. Aging Dis. 2019;10:1159–73.
Bakshi R, Xu Y, Mueller KA, Chen X, Granucci E, Paganoni S, et al. Urate mitigates oxidative stress and motor neuron toxicity of astrocytes derived from ALS-linked SOD1(G93A) mutant mice. Mol Cell Neurosci. 2018;92:12–6.
Allen SP, Hall B, Castelli LM, Francis L, Woof R, Siskos AP, et al. Astrocyte adenosine deaminase loss increases motor neuron toxicity in amyotrophic lateral sclerosis. Brain. 2019;142:586–605.
Zhou C, Zhong W, Zhou J, Sheng F, Fang Z, Wei Y, et al. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy. 2012;8:1215–26.
Durrenberger PF, Fernando FS, Kashefi SN, Bonnert TP, Seilhean D, Nait-Oumesmar B, et al. Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transm (Vienna). 2015;122:1055–68.
Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–7.
Chen M, Xu S, Zhou P, He G, Jie Q, Wu Y. Desloratadine citrate disodium injection, a potent histamine H(1) receptor antagonist, inhibits chemokine production in ovalbumin-induced allergic rhinitis guinea pig model and histamine-induced human nasal epithelial cells via inhibiting the ERK1/2 and NF-kappa B signal cascades. Eur J Pharmacol. 2015;767:98–107.
Barber SC, Mead RJ, Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006;1762:1051–67.
Bujak AL, Crane JD, Lally JS, Ford RJ, Kang SJ, Rebalka IA, et al. AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metab. 2015;21:883–90.
Xiao L, Liu J, Sun Z, Yin Y, Mao Y, Xu D, et al. AMPK-dependent and -independent coordination of mitochondrial function and muscle fiber type by FNIP1. PLoS Genet. 2021;17:e1009488.
Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92.
Zhang H, Li H, Huang B, Wang S, Gao Y, Meng F, et al. Spatiotemporal evolution of pyroptosis and canonical inflammasome pathway in hSOD1(G93A) ALS mouse model. BMC Neurosci. 2022;23:50.
Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 2017;170:649–63. e13.
Cocco S, Leone A, Roca MS, Lombardi R, Piezzo M, Caputo R, et al. Inhibition of autophagy by chloroquine prevents resistance to PI3K/AKT inhibitors and potentiates their antitumor effect in combination with paclitaxel in triple negative breast cancer models. J Transl Med. 2022;20:290.
Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109:17–23.
Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5-HT(2A) receptor agonist TCB-2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl). 2010;212:13–23.
Lv J, Wang W, Zhu X, Xu X, Yan Q, Lu J, et al. DW14006 as a direct AMPKα1 activator improves pathology of AD model mice by regulating microglial phagocytosis and neuroinflammation. Brain Behav Immun. 2020;90:55–69.
Li M, Xu T, Zhou F, Wang M, Song H, Xiao X, et al. Neuroprotective effects of four phenylethanoid glycosides on H2O2-induced apoptosis on PC12 cells via the Nrf2/ARE pathway. Int J Mol Sci. 2018;19:1135.
Petri S, Korner S, Kiaei M. Nrf2/ARE signaling pathway: key mediator in oxidative stress and potential therapeutic target in ALS. Neurol Res Int. 2012;2012:878030.
Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68:185–97. e6.
Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22.
Mann CN, Devi SS, Kersting CT, Bleem AV, Karch CM, Holtzman DM, et al. Astrocytic alpha2-Na+/K+ ATPase inhibition suppresses astrocyte reactivity and reduces neurodegeneration in a tauopathy mouse model. Sci Transl Med. 2022;14:eabm4107.
Yamanaka K, Komine O. The multi-dimensional roles of astrocytes in ALS. Neurosci Res. 2018;126:31–8.
Arredondo C, Cefaliello C, Dyrda A, Jury N, Martinez P, Diaz I, et al. Excessive release of inorganic polyphosphate by ALS/FTD astrocytes causes non-cell-autonomous toxicity to motoneurons. Neuron. 2022;110:1656–70. e12.
Evans CS, Holzbaur ELF. Autophagy and mitophagy in ALS. Neurobiol Dis. 2019;122:35–40.
Meyer M, Lima A, Deniselle MCG, De Nicola AF. Early signs of neuroinflammation in the postnatal wobbler mouse model of amyotrophic lateral sclerosis. Cell Mol Neurobiol. 2023;43:2149–63.
Garofalo S, Cocozza G, Bernardini G, Savage J, Raspa M, Aronica E, et al. Blocking immune cell infiltration of the central nervous system to tame neuroinflammation in amyotrophic lateral sclerosis. Brain Behav Immun. 2022;105:1–14.
Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–52.
Hu H, Tan CC, Tan L, Yu JT. A mitocentric view of Alzheimer’s disease. Mol Neurobiol. 2017;54:6046–60.
Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA. ALS genetics, mechanisms, and therapeutics: where are we now? Front Neurosci. 2019;13:1310.
Meyer T. [Amyotrophic lateral sclerosis (ALS) - diagnosis, course of disease and treatment options]. Dtsch Med Wochenschr. 2021;146:1613–8.
Modol-Caballero G, Garcia-Lareu B, Herrando-Grabulosa M, Verdes S, Lopez-Vales R, Pages G, et al. Specific expression of glial-derived neurotrophic factor in muscles as gene therapy strategy for amyotrophic lateral sclerosis. Neurotherapeutics. 2021;18:1113–26.
Spaulding HR, Yan Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022;84:209–27.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82273930), the National Natural Science Foundation for Young Scientists of China (82304468, 82304494, 82204486), Major Program of the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (23KJA350002), the Natural Science Foundation for Young Scientists of Nanjing University of Chinese Medicine (XPT82204486), Natural Science Foundation of Jiangsu Province (BK20200570).
Author information
Authors and Affiliations
Contributions
XS, JYW and JL designed the study. XS reviewed the manuscript. JL, ZYJ, AXH, MZ, ZXL, FZ and LM performed the animal and cell experiments. JL, ZYJ and AXH analyzed and interpreted data. JL wrote the manuscript. JL, XS, JYW and HMJ are the guarantors of this work and, as such, have full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. All institutional and national guidelines for the care and use of laboratory animals were followed.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., He, Ax., Jin, Zy. et al. Desloratadine alleviates ALS-like pathology in hSOD1G93A mice via targeting 5HTR2A on activated spinal astrocytes. Acta Pharmacol Sin 45, 926–944 (2024). https://doi.org/10.1038/s41401-023-01223-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01223-2