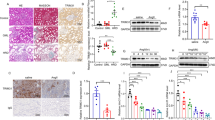
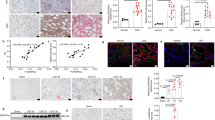
Abstract
Hypertensive renal disease (HRD) contributes to the progression of kidney dysfunction and ultimately leads to end-stage renal disease. Understanding the mechanisms underlying HRD is critical for the development of therapeutic strategies. Deubiquitinating enzymes (DUBs) have been recently highlighted in renal pathophysiology. In this study, we investigated the role of a DUB, OTU Domain-Containing Protein 1 (OTUD1), in HRD models. HRD was induced in wild-type or Otud1 knockout mice by chronic infusion of angiotensin II (Ang II, 1 μg/kg per min) through a micro-osmotic pump for 4 weeks. We found that OTUD1 expression levels were significantly elevated in the kidney tissues of Ang II-treated mice. Otud1 knockout significantly ameliorated Ang II-induced HRD, whereas OTUD1 overexpression exacerbated Ang II-induced kidney damage and fibrosis. Similar results were observed in TCMK-1 cells but not in SV40 MES-13 cells following Ang II (1 μM) treatment. In Ang II-challenged TCMK-1 cells, we demonstrated that OTUD1 bound to CDK9 and induced CDK9 deubiquitination: OTUD1 catalyzed K63 deubiquitination on CDK9 with its Cys320 playing a critical role, promoting CDK9 phosphorylation and activation to induce inflammatory responses and fibrosis in kidney epithelial cells. Administration of a CDK9 inhibitor NVP-2 significantly ameliorated Ang II-induced HRD in mice. This study demonstrates that OTUD1 mediates HRD by targeting CDK9 in kidney epithelial cells, suggesting OTUD1 is a potential target in treating this disease.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan A, Lasserson DS, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11:e0158765.
Rai A, Narisawa M, Li P, Piao LM, Li YL, Yang G, et al. Adaptive immune disorders in hypertension and heart failure: focusing on T-cell subset activation and clinical implications. J Hypertens. 2020;38:1878–89.
Zhang J, Cao L, Wang XH, Li Q, Zhang M, Cheng C, et al. The E3 ubiquitin ligase TRIM31 plays a critical role in hypertensive nephropathy by promoting proteasomal degradation of MAP3K7 in the TGF-β1 signaling pathway. Cell Death Differ. 2022;29:556–67.
Zhou LL, Li YJ, Hao S, Zhou D, Tan RJ, Nie J, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 2015;26:107–20.
Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–19.
Ihara Y, Morishima-Kawashima M, Nixon R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer’s disease. Cold Spring Harb Perspect Med. 2012;2:8.
Roberts JZ, Crawford N, Longley DB. The role of ubiquitination in apoptosis and necroptosis. Cell Death Differ. 2022;29:272–84.
Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605.
Oikawa D, Shimizu K, Tokunaga F. Pleiotropic roles of a KEAP1-associated deubiquitinase, OTUD1. Antioxidants. 2023;12:2.
Song J, Liu TT, Yin Y, Zhao W, Lin ZQ, Yin YX, et al. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021;22:e51162.
Wang MY, Han X, Yu TX, Wang MX, Luo W, Zou CP, et al. OTUD1 promotes pathological cardiac remodeling and heart failure by targeting STAT3 in cardiomyocytes. Theranostics. 2023;13:2263–80.
Huang ZQ, Shen SR, Wang MY, Li WX, Wu GJ, Huang WJ, et al. Mouse endothelial OTUD1 promotes angiotensin II-induced vascular remodeling by deubiquitinating SMAD3. EMBO Rep. 2023;24:e56135.
Guhan SM, Shaughnessy M, Rajadurai A, Taylor M, Kumar R, Ji ZY, et al. The molecular context of vulnerability for CDK9 suppression in triple wild-type melanoma. J Invest Dermatol. 2021;141:2018–27.
Olson CM, Jiang BS, Erb MA, Liang YK, Doctor ZM, Zhang ZN, et al. Pharmacological perturbation of CDK9 using selective CDK9 inhibition or degradation. Nat Chem Biol. 2018;14:163–70.
Zhang ZM, Wang DD, Wang PY, Zhao YC, You FP. OTUD1 negatively regulates type I IFN induction by disrupting noncanonical ubiquitination of IRF3. J Immunol. 2020;204:1904–18.
Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80.
Restrepo JM, Torres-Canchala L, Bonventre JV, Arias JC, Ferguson M, Villegas A, et al. Urinary KIM-1 is not correlated with gestational age among 5-year-old children born prematurely. Front Pediatr. 2023;11:1038206.
Karmakova ТА, Sergeeva NS, Kanukoev KY, Alekseev BY, Kaprin АD. Kidney injury molecule 1 (KIM-1): a multifunctional glycoprotein and biological marker (Review). Sovrem Tekhnologii Med. 2021;13:64–78.
Qu XL, Jiang MJ, Sun YB, Jiang XY, Fu P, Ren Y, et al. The Smad3/Smad4/CDK9 complex promotes renal fibrosis in mice with unilateral ureteral obstruction. Kidney Int. 2015;88:1323–35.
Yang XJ, Luo W, Li L, Hu X, Xu MJ, Wang Y, et al. CDK9 inhibition improves diabetic nephropathy by reducing inflammation in the kidneys. Toxicol Appl Pharmacol 2021;416:115465.
Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102.
Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5.
Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–86.
Zhang Y, Hu RM, Wu HJ, Jiang WN, Sun Y, Wang Y, et al. OTUB1 overexpression in mesangial cells is a novel regulator in the pathogenesis of glomerulonephritis through the decrease of DCN level. PLoS One. 2012;7:e29654.
Park JH, Kim SY, Cho HJ, Lee SY, Baek KH. YOD1 Deubiquitinates NEDD4 involved in the Hippo signaling pathway. Cell Physiol Biochem. 2020;54:1–14.
Zhao Y, Chen X, Lin YM, Li ZD, Su X, Fan SJ, et al. USP25 inhibits renal fibrosis by regulating TGFβ-SMAD signaling pathway in Ang II-induced hypertensive mice. Biochim Biophys Acta Mol Basis Dis. 2023;1869:166713.
Liu WT, Yan B, Yu HX, Ren JN, Peng M, Zhu L, et al. OTUD1 stabilizes PTEN to inhibit the PI3K/AKT and TNF-alpha/NF-kappaB signaling pathways and sensitize ccRCC to TKIs. Int J Biol Sci. 2022;18:1401–14.
Vdovin A, Jelinek T, Zihala D, Sevcikova T, Durech M, Sahinbegovic H, et al. The deubiquitinase OTUD1 regulates immunoglobulin production and proteasome inhibitor sensitivity in multiple myeloma. Nat Commun. 2022;13:6820.
Huang Z, Wang TQ, Wang C, Fan Y. CDK9 inhibitors in cancer research. RSC Med Chem. 2022;13:688–710.
Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–35.
Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nat Struct Biol. 1996;3:696–700.
Ramakrishnan R, Dow EC, Rice AP. Characterization of Cdk9 T-loop phosphorylation in resting and activated CD4+ T lymphocytes. J Leukoc Biol. 2009;86:1345–50.
Fu JJ, Yoon HG, Qin J, Wong JM. Regulation of P-TEFb elongation complex activity by CDK9 acetylation. Mol Cell Biol. 2007;27:4641–51.
Mbonye U, Wang BL, Gokulrangan G, Shi WX, Yang SC, Karn J. Cyclin-dependent kinase 7 (CDK7)-mediated phosphorylation of the CDK9 activation loop promotes P-TEFb assembly with Tat and proviral HIV reactivation. J Biol Chem. 2018;293:10009–25.
Oikawa D, Gi M, Kosako H, Shimizu K, Takahashi H, Shiota M, et al. OTUD1 deubiquitinase regulates NF-κB- and KEAP1-mediated inflammatory responses and reactive oxygen species-associated cell death pathways. Cell Death Dis. 2022;13:694.
Wu B, Qiang LH, Zhang Y, Fu YS, Zhao MY, Lei ZH, et al. The deubiquitinase OTUD1 inhibits colonic inflammation by suppressing RIPK1-mediated NF-κB signaling. Cell Mol Immunol. 2022;19:276–89.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82000793 to WL and 82370829 for HZ) and the Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A) at Hangzhou Medical College.
Author information
Authors and Affiliations
Contributions
GL, HZ, and WL contributed to the literature search and study design. MYW, TXY, QYW, XH, SJY, and XH carried out the experiments. WL and MYW contributed to data collection and analysis. MYW and WL participated in the drafting of the article. GL, YW, and XHL revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, My., Yu, Tx., Wang, Qy. et al. OTUD1 promotes hypertensive kidney fibrosis and injury by deubiquitinating CDK9 in renal epithelial cells. Acta Pharmacol Sin 45, 765–776 (2024). https://doi.org/10.1038/s41401-023-01192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01192-6