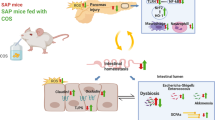
Abstract
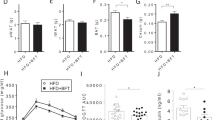
Amuc_1100 is a membrane protein from Akkermansia muciniphila, which has been found to play a role in host immunological homeostasis in the gastrointestinal tract by activating TLR2 and TLR4. In this study we investigated the effects and underlying mechanisms of Amuc_1100 on acute pancreatitis (AP) induced in mice by intraperitoneal injection of caerulein and lipopolysaccharide (LPS). The mice were treated with the protein Amuc_1100 (3 μg, i.g.) for 20 days before caerulein injection. Cecal contents of the mice were collected for 16S rRNA sequencing. We found that pretreatment with Amuc_1100 significantly alleviated AP-associated pancreatic injury, reduced serum amylase and lipase. Amuc_1100 pretreatment significantly inhibited the expression of proinflammatory cytokines (TNF-α, IL-1β, IFN-γ and IL-6) in spleen and pancreas through inhibiting NF-κB signaling pathway. Moreover, Amuc_1100 pretreatment significantly decreased the inflammatory infiltration, accompanied by the reduction of Ly6C+ macrophages and neutrophils in the spleen of AP mice. Gut microbiome analysis showed that the abundance of Bacteroidetes, Proteobacteria, Desulfobacterota and Campilobacterota was decreased, while the proportion of Firmicutes and Actinobacteriota was increased in AP mice pretreated with Amuc_1100. We further demonstrated that Amuc_1100 pretreatment restored the enrichment of tryptophan metabolism, which was mediated by intestinal flora. These results provide new evidence that Amuc_1100 lessens the severity of AP through its anti-inflammatory properties with a reduction of macrophages and neutrophil infiltration, as well as its regulation of the composition of intestinal flora and tryptophan metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gardner TB. Acute pancreatitis. Ann Intern Med. 2021;174:17–32.
Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375:1972–81.
Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The changing epidemiology of acute pancreatitis hospitalizations: a decade of trends and the impact of chronic pancreatitis. Pancreas. 2017;46:482–8.
Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85–96.
Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396:726–34.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11.
Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154:1103–39.
Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325:382–90.
Patel BK, Patel KH, Bhatia M, Iyer SG, Madhavan K, Moochhala SM. Gut microbiome in acute pancreatitis: A review based on current literature. World J Gastroenterol. 2021;27:5019–36.
Schmitt FCF, Brenner T, Uhle F, Loesch S, Hackert T, Ulrich A, et al. Gut microbiome patterns correlate with higher postoperative complication rates after pancreatic surgery. BMC Microbiol. 2019;19:42.
Malla MA, Dubey A, Kumar A, Yadav S, Hashem A, Abd Allah EF. Exploring the human microbiome: the potential future role of next-generation sequencing in disease diagnosis and treatment. Front Immunol. 2018;9:2868.
Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94:441–8.
Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, et al. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas. 2015;44:868–75.
Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, et al. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery. 2009;145:157–67.
Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y, et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 2019;54:347–58.
Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–13.
Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12:e0173004.
Wang J, Xiang R, Wang R, Zhang B, Gong W, Zhang J, et al. The variable oligomeric state of Amuc_1100 from Akkermansia muciniphila. J Struct Biol. 2020;212:107593.
Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut. 2020;69:1988–97.
Mulhall H, DiChiara JM, Deragon M, Iyer R, Huck O, Amar S. Akkermansia muciniphila and its pili-like protein Amuc_1100 modulate macrophage polarization in experimental periodontitis. Infect Immun. 2020;89:e00500–20.
Wang J, Xu W, Wang R, Cheng R, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021;12:3597–610.
Cani PD, Knauf C. A newly identified protein from Akkermansia muciniphila stimulates GLP-1 secretion. Cell Metab. 2021;33:1073–5.
Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765.
Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284–8.
Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflamm-aging. Ageing Res Rev. 2008;7:83–105.
Piao X, Liu B, Sui X, Li S, Niu W, Zhang Q, et al. Picroside II improves severe acute pancreatitis-induced intestinal barrier injury by inactivating oxidative and inflammatory TLR4-dependent PI3K/AKT/NF-κB signaling and improving gut microbiota. Oxid Med Cell Longev. 2020;2020:3589497.
Liu K, Yang L, Wang X, Huang Q, Tuerhong K, Yang M, et al. Electroacupuncture regulates macrophage, neutrophil, and oral microbiota to alleviate alveolar bone loss and inflammation in experimental ligature-induced periodontitis. J Clin Periodontol. 2023;50:368–79.
Mulhall H, DiChiara JM, Huck O, Amar S. Pasteurized Akkermansia muciniphila reduces periodontal and systemic inflammation induced by Porphyromonas gingivalis in lean and obese mice. J Clin Periodontol. 2022;49:717–29.
Xu X, Qiao Y, Peng Q, Dia VP, Shi B. Probiotic activity of ropy Lactiplantibacillus plantarum NA isolated from Chinese northeast sauerkraut and comparative evaluation of its live and heat-killed cells on antioxidant activity and RAW 264.7 macrophage stimulation. Food Funct. 2023;14:2481–95.
Shi R, Wang J, Zhang Z, Leng Y, Chen AF. ASGR1 promotes liver injury in sepsis by modulating monocyte-to-macrophage differentiation via NF-κB/ATF5 pathway. Life Sci. 2023;315:121339.
Van Gassen N, Van Overmeire E, Leuckx G, Heremans Y, De Groef S, Elkrim Y, et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. Eur J Immunol. 2015;45:1482–93.
He J, Wang K, Liu M, Zeng W, Li D, Majigsuren Z, et al. β-hydroxybutyrate enhances bovine neutrophil adhesion by inhibiting autophagy. Front Immunol. 2022;13:1096813.
Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg. 2003;7:26–36.
Zhang D, Zhu C, Fang Z, Zhang H, Yang J, Tao K, et al. Remodeling gut microbiota by Clostridium butyricum (C. butyricum) attenuates intestinal injury in burned mice. Burns. 2020;46:1373–80.
Shin J, Noh JR, Chang DH, Kim YH, Kim MH, Lee ES, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10:1137.
Dong X, Liu M, Liu X, Liu M, Zhang X, Wang G. Prokaryotic expression of Amuc_1100 protein and its effects on high-fat diet rats combined streptozotocin injection. Wei Sheng Yan Jiu. 2020;49:785–822.
Liu J, Luo M, Qin S, Li B, Huang L, Xia X. Significant succession of intestinal bacterial community and function during the initial 72 h of acute pancreatitis in rats. Front Cell Infect Microbiol. 2022;12:808991.
Li Q, Wang C, Tang C, Zhao X, He Q, Li J. Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front Cell Infect Microbiol. 2018;8:5.
Oh SJ, Pimentel M, Leite GGS, Celly S, Villanueva-Millan MJ, Lacsina I, et al. Acute appendicitis is associated with appendiceal microbiome changes including elevated Campylobacter jejuni levels. BMJ Open Gastroenterol. 2020;7:e000412.
Khan I, Yasir M, Azhar EI, Kumosani T, Barbour EK, Bibi F, et al. Implication of gut microbiota in human health. CNS Neurol Disord Drug Targets. 2014;13:1325–33.
Guo J, Li X, Wang D, Guo Y, Cao T. Exploring metabolic biomarkers and regulation pathways of acute pancreatitis using ultra-performance liquid chromatography combined with a mass spectrometry-based metabolomics strategy. RSC Adv. 2019;9:12162–73.
Zhang K, Li X, Wang X, Zheng H, Tang S, Lu L, et al. Gut barrier proteins mediate liver regulation by the effects of serotonin on the non-alcoholic fatty liver disease. Curr Protein Pept Sci. 2020;21:978–84.
Li X, Zhang ZH, Zabed HM, Yun J, Zhang G, Qi X. An insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol Nutr Food Res. 2021;65:e2000461.
Skouras C, Zheng X, Binnie M, Homer NZ, Murray TB, Robertson D, et al. Increased levels of 3-hydroxykynurenine parallel disease severity in human acute pancreatitis. Sci Rep. 2016;6:33951.
Acknowledgements
The authors acknowledge the science and technology experiment center in Nanjing University of Chinese Medicine for flow cytometry.
Funding
This project was financially supported by the National Natural Science Foundations of China (No. 32270192 and 82072240), the Open Funding Project of the State Key Laboratory of Bioreactor Engineering, the Open Project of the State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences (No. SIMM2205KF-15), the Open Project of Chinese Materia Medica First-Class Discipline of Nanjing University of Chinese Medicine (No. 2020YLXK008), the Natural Science Foundation of Jiangsu Province of China (BK20230064 to WL), the Fok Ying Tung Education Foundation (No. 171033 to WL), high level key discipline construction project of the National Administration of Traditional Chinese Medicine-Resource Chemistry of Chinese Medicinal Materials (No. zyyzdxk-2023083 to WL).
Author information
Authors and Affiliations
Contributions
WL and LJW directed the research and wrote the manuscript. LJW designed the experiments and WLP analyzed the data. YLJ carried out most of the experiments. JCL helped with raising mice. HE staining was carried out by RLZ. JJW performed some Western blot experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Lj., Jin, Yl., Pei, Wl. et al. Amuc_1100 pretreatment alleviates acute pancreatitis in a mouse model through regulating gut microbiota and inhibiting inflammatory infiltration. Acta Pharmacol Sin 45, 570–580 (2024). https://doi.org/10.1038/s41401-023-01186-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01186-4