Abstract
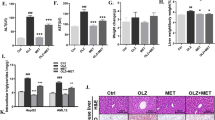
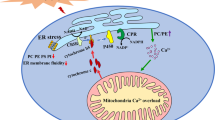
Olanzapine (OLZ) is a widely prescribed antipsychotic drug with a relatively ideal effect in the treatment of schizophrenia (SCZ). However, its severe metabolic side effects often deteriorate clinical therapeutic compliance and mental rehabilitation. The peripheral mechanism of OLZ-induced metabolic disorders remains abstruse for its muti-target activities. Endoplasmic reticulum (ER) stress is implicated in cellular energy metabolism and the progression of psychiatric disorders. In this study, we investigated the role of ER stress in the development of OLZ-induced dyslipidemia. A cohort of 146 SCZ patients receiving OLZ monotherapy was recruited, and blood samples and clinical data were collected at baseline, and in the 4th week, 12th week, and 24th week of the treatment. This case-control study revealed that OLZ treatment significantly elevated serum levels of endoplasmic reticulum (ER) stress markers GRP78, ATF4, and CHOP in SCZ patients with dyslipidemia. In HepG2 cells, treatment with OLZ (25, 50 μM) dose-dependently enhanced hepatic de novo lipogenesis accompanied by SREBPs activation, and simultaneously triggered ER stress. Inhibition of ER stress by tauroursodeoxycholate (TUDCA) and 4-phenyl butyric acid (4-PBA) attenuated OLZ-induced lipid dysregulation in vitro and in vivo. Moreover, we demonstrated that activation of PERK-CHOP signaling during ER stress was a major contributor to OLZ-triggered abnormal lipid metabolism in the liver, suggesting that PERK could be a potential target for ameliorating the development of OLZ-mediated lipid dysfunction. Taken together, ER stress inhibitors could be a potentially effective intervention against OLZ-induced dyslipidemia in SCZ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50.
Jauhar S, Johnstone M, Mckenna PJ. Schizophrenia. Lancet. 2022;399:473–86.
Ceraso A, Lin JJ, Schneider-Thoma J, Siafis S, Tardy M, Komossa K, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;2020:Cd008016.
Cernea S, Dima L, Correll CU, Manu P. Pharmacological management of glucose dysregulation in patients treated with second-generation antipsychotics. Drugs. 2020;80:1763–81.
Wada M, Noda Y, Iwata Y, Tsugawa S, Yoshida K, Tani H, et al. Dopaminergic dysfunction and excitatory/inhibitory imbalance in treatment-resistant schizophrenia and novel neuromodulatory treatment. Mol Psychiatry. 2022;27:2950–67.
Lähteenvuo M, Tiihonen J. Antipsychotic polypharmacy for the management of schizophrenia: evidence and recommendations. Drugs. 2021;81:1273–84.
Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–64.
Carli M, Kolachalam S, Longoni B, Pintaudi A, Baldini M, Aringhieri S, et al. Atypical antipsychotics and metabolic syndrome: from molecular mechanisms to clinical differences. Pharmaceuticals. 2021;14:238.
Mazereel V, Detraux J, Vancampfort D, Van Winkel R, De Hert M. Impact of psychotropic medication effects on obesity and the metabolic syndrome in people with serious mental illness. Front Endocrinol. 2020;11:573479.
Vantaggiato C, Panzeri E, Citterio A, Orso G, Pozzi M. Antipsychotics promote metabolic disorders disrupting cellular lipid metabolism and trafficking. Trends Endocrinol Metab. 2019;30:189–210.
Rojo LE, Gaspar PA, Silva H, Risco L, Arena P, Cubillos-Robles K, et al. Metabolic syndrome and obesity among users of second generation antipsychotics: A global challenge for modern psychopharmacology. Pharmacol Res. 2015;101:74–85.
Ono S, Sugai T, Suzuki Y, Yamazaki M, Shimoda K, Mori T, et al. High-density lipoprotein-cholesterol and antipsychotic medication in overweight inpatients with schizophrenia: post-hoc analysis of a Japanese nationwide survey. BMC Psychiatry. 2018;18:1–7.
Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. 2019;76:1052–62.
Ferreira V, Folgueira C, Guillén M, Zubiaur P, Navares M, Sarsenbayeva A, et al. Modulation of hypothalamic AMPK phosphorylation by olanzapine controls energy balance and body weight. Metabolism. 2022;137:155335.
Huang J, Hei GR, Yang Y, Liu CC, Xiao JM, Long YJ, et al. Increased appetite plays a key role in olanzapine-induced weight gain in first-episode schizophrenia patients. Front Pharmacol. 2020;11:739.
Handen BL, Anagnostou E, Aman MG, Sanders KB, Chan J, Hollway JA, et al. A randomized, placebo-controlled trial of metformin for the treatment of overweight induced by antipsychotic medication in young people with autism spectrum disorder: open-label extension. J Am Acad Child Adolesc Psychiatry. 2017;56:849–56.
Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind, placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PLoS One. 2011;6:e22662.
Hahn MK, Wolever TM, Arenovich T, Teo C, Giacca A, Powell V, et al. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J Clin Psychopharmacol. 2013;33:740–6.
Vucicevic L, Misirkic-Marjanovic M, Paunovic V, Kravic-Stevovic T, Martinovic T, Ciric D, et al. Autophagy inhibition uncovers the neurotoxic action of the antipsychotic drug olanzapine. Autophagy. 2014;10:2362–78.
Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat Rev Endocrinol. 2017;13:710–30.
Liu X, Lian J, Hu CH, Deng C. Betahistine co-treatment ameliorates dyslipidemia induced by chronic olanzapine treatment in rats through modulation of hepatic AMPKα-SREBP-1 and PPARα-dependent pathways. Pharmacol Res. 2015;100:36–46.
Lian J, Huang XF, Pai N, Deng C. Ameliorating antipsychotic-induced weight gain by betahistine: mechanisms and clinical implications. Pharmacol Res. 2016;106:51–63.
Liu X, Deng C, Cao S, Gong J, Wang BC, Hu CH. Acute effects of oral olanzapine treatment on the expression of fatty acid and cholesterol metabolism-related gene in rats. Life Sci. 2015;128:72–8.
Raeder MB, Fernø J, Vik-Mo AO, Steen VM. SREBP activation by antipsychotic-and antidepressant-drugs in cultured human liver cells: relevance for metabolic side-effects. Mol Cell Biochem. 2006;289:167–73.
Yang LH, Chen TM, Yu ST, Chen YH. Olanzapine induces SREBP-1-related adipogenesis in 3T3-L1 cells. Pharmacol Res. 2007;56:202–8.
Polymeropoulos MH, Licamele L, Volpi S, Mack K, Mitkus SN, Carstea ED, et al. Common effect of antipsychotics on the biosynthesis and regulation of fatty acids and cholesterol supports a key role of lipid homeostasis in schizophrenia. Schizophr Res. 2009;108:134–42.
Skrede S, Fernø J, Vázquez MJ, Fjær S, Pavlin T, Lunder N, et al. Olanzapine, but not aripiprazole, weight-independently elevates serum triglycerides and activates lipogenic gene expression in female rats. Int J Neuropsychopharmacol. 2012;15:163–79.
Le Hellard S, Mühleisen T, Djurovic S, Fernø J, Ouriaghi Z, Mattheisen M, et al. Polymorphisms in SREBF1 and SREBF2, two antipsychotic-activated transcription factors controlling cellular lipogenesis, are associated with schizophrenia in German and Scandinavian samples. Mol Psychiatry. 2010;15:463–72.
Vassas TJ, Burghardt KJ, Ellingrod VL. Pharmacogenomics of sterol synthesis and statin use in schizophrenia subjects treated with antipsychotics. Pharmacogenomics. 2014;15:61–7.
Yang L, Chen J, Liu D, Yu S, Cong E, Li Y, et al. Association between SREBF2 gene polymorphisms and metabolic syndrome in clozapine-treated patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:136–41.
Debose-Boyd RA, Ye J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem Sci. 2018;43:358–68.
Xu D, Wang Z, Xia Y, Shao F, Xia W, Wei Y, et al. The gluconeogenic enzyme PCK1 phosphorylates INSIG1/2 for lipogenesis. Nature. 2020;580:530–5.
Kim JY, Garcia-Carbonell R, Yamachika S, Zhao P, Dhar D, Loomba R, et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell. 2018;175:133–45.
Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, et al. Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab. 2021;33:319–33.
Kawamura S, Matsushita Y, Kurosaki S, Tange M, Fujiwara N, Hayata Y, et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis. J Clin Invest. 2022;132:e151895.
Kim JY, Wang LQ, Sladky VC, Oh TG, Liu J, Trinh K, et al. PIDDosome-SCAP crosstalk controls high-fructose-diet-dependent transition from simple steatosis to steatohepatitis. Cell Metab. 2022;34:1548–60.
Zheng ZG, Zhu ST, Cheng HM, Zhang X, Cheng G, Thu PM, et al. Discovery of a potent SCAP degrader that ameliorates HFD-induced obesity, hyperlipidemia and insulin resistance via an autophagy-independent lysosomal pathway. Autophagy. 2021;17:1592–613.
Patel S, Sharma D, Kalia K, Tiwari V. Crosstalk between endoplasmic reticulum stress and oxidative stress in schizophrenia: The dawn of new therapeutic approaches. Neurosci Biobehav Rev. 2017;83:589–603.
Zhou R, He M, Fan J, Li R, Zuo Y, Li B, et al. The role of hypothalamic endoplasmic reticulum stress in schizophrenia and antipsychotic-induced weight gain: a narrative review. Front Neurosci. 2022;16:947295.
He M, Huang XF, Gao G, Zhou T, Li W, Hu J, et al. Olanzapine-induced endoplasmic reticulum stress and inflammation in the hypothalamus were inhibited by an ER stress inhibitor 4-phenylbutyrate. Psychoneuroendocrinology. 2019;104:286–99.
Chen J, Wu H, Tang X, Chen L. 4-Phenylbutyrate protects against rifampin-induced liver injury via regulating MRP2 ubiquitination through inhibiting endoplasmic reticulum stress. Bioengineered. 2022;13:2866–77.
Kim YR, Lee EJ, Shin KO, Kim MH, Pewzner-Jung Y, Lee YM, et al. Hepatic triglyceride accumulation via endoplasmic reticulum stress-induced SREBP-1 activation is regulated by ceramide synthases. Exp Mol Med. 2019;51:1–16.
Ma Y, Zhang M, Yu H, Lu J, Cheng KK, Zhou J, et al. Activation of G0/G1 switch gene 2 by endoplasmic reticulum stress enhances hepatic steatosis. Metabolism. 2019;99:32–44.
Liu XM, Zhao XM, Deng C, Zeng YP, Hu CH. Simvastatin improves olanzapine-induced dyslipidemia in rats through inhibiting hepatic mTOR signaling pathway. Acta Pharmacol Sin. 2019;40:1049–57.
Brand BA, De Boer JN, Dazzan P, Sommer IE. Towards better care for women with schizophrenia-spectrum disorders. Lancet Psychiatry. 2022;9:330–6.
Seeman MV. Men and women respond differently to antipsychotic drugs. Neuropharmacology. 2020;163:107631.
Girona J, Rodríguez-Borjabad C, Ibarretxe D, Vallvé JC, Ferré R, Heras M, et al. The circulating GRP78/BiP is a marker of metabolic diseases and atherosclerosis: bringing endoplasmic reticulum stress into the clinical scenario. J Clin Med. 2019;8:1793.
Paris EA, Bahr JM, Abramowicz JS, Basu S, Barua A. Glucose-regulated protein 78 is a potential serum and imaging marker for early detection of ovarian cancer. Cancers. 2023;15:1140.
Sabirli R, Koseler A, Goren T, Turkcuer I, Kurt O. High GRP78 levels in Covid-19 infection: a case-control study. Life Sci. 2021;265:118781.
Kristiana I, Sharpe L, Catts V, Lutze-Mann L, Brown A. Antipsychotic drugs upregulate lipogenic gene expression by disrupting intracellular trafficking of lipoprotein-derived cholesterol. Pharmacogenomics J. 2010;10:396–407.
Su Y, Liu X, Lian J, Deng C. Epigenetic histone modulations of PPARγ and related pathways contribute to olanzapine-induced metabolic disorders. Pharmacol Res. 2020;155:104703.
Lemmer IL, Willemsen N, Hilal N, Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol Metab. 2021;47:101169.
Song Q, Chen Y, Wang J, Hao L, Huang C, Griffiths A, et al. ER stress-induced upregulation of NNMT contributes to alcohol-related fatty liver development. J Hepatol. 2020;73:783–93.
Matteoni S, Matarrese P, Ascione B, Ricci-Vitiani L, Pallini R, Villani V, et al. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J Exp Clin Cancer Res. 2021;40:347.
Shi L, Chen H, Chen K, Zhong C, Song C, Huang Y, et al. The DRD2 antagonist haloperidol mediates autophagy-induced ferroptosis to increase temozolomide sensitivity by promoting endoplasmic reticulum stress in glioblastoma. Clin Cancer Res. 2023;29:3172–88.
Weston-Green K, Babic I, De Santis M, Pan B, Montgomery MK, Mitchell T, et al. Disrupted sphingolipid metabolism following acute clozapine and olanzapine administration. J Biomed Sci. 2018;25:1–11.
Chattopadhyay A, Guan P, Majumder S, Kaw K, Zhou Z, Zhang C, et al. Preventing cholesterol-induced perk (protein kinase RNA-like endoplasmic reticulum kinase) signaling in smooth muscle cells blocks atherosclerotic plaque formation. Arterioscler Thromb Vasc Biol. 2022;42:1005–22.
Chen S, Henderson A, Petriello MC, Romano KA, Gearing M, Miao J, et al. Trimethylamine N-oxide binds and activates PERK to promote metabolic dysfunction. Cell Metab. 2019;30:1141–51.
Wang B, Zhang M, Urabe G, Huang Y, Chen G, Wheeler D, et al. PERK inhibition mitigates restenosis and thrombosis: a potential low-thrombogenic antirestenotic paradigm. JACC Basic Transl Sci. 2020;5:245–63.
Correll CU, Newcomer JW, Silverman B, Dipetrillo L, Graham C, Jiang Y, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177:1168–78.
Correll CU, Stein E, Graham C, Dipetrillo L, Akerman S, Stanford AD, et al. Reduction in multiple cardiometabolic risk factors with combined olanzapine/samidorphan compared with olanzapine: post hoc analyses from a 24-week phase 3 study. Schizophr Bull. 2023;49:454–63.
Imbernon M, Sanchez-Rebordelo E, Romero-Picó A, Kalló I, Chee MJ, Porteiro B, et al. Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology. 2016;64:1086–104.
Acknowledgements
This study was supported by the Chongqing Basic Research and Frontier Exploration Project (cstc2022ycjh-bgzxm0119).
Author information
Authors and Affiliations
Contributions
CHH and LL conducted the literature searches and designed the experiments; JML, LT, SYC, CYY, and LL collected patient blood samples and clinical data; LL conducted the experiments and drafted the article in this study; CHH, XML, and LL analyzed and interpreted data, and revised the article; All authors approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Tang, L., Luo, Jm. et al. Activation of the PERK-CHOP signaling pathway during endoplasmic reticulum stress contributes to olanzapine-induced dyslipidemia. Acta Pharmacol Sin 45, 502–516 (2024). https://doi.org/10.1038/s41401-023-01180-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01180-w
Keywords
This article is cited by
-
Updated mechanisms of MASLD pathogenesis
Lipids in Health and Disease (2024)