Abstract
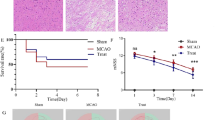
There are few effective and safe neuroprotective agents for the treatment of ischemic stroke currently. Caffeic acid is a phenolic acid that widely exists in a number of plant species. Previous studies show that caffeic acid ameliorates brain injury in rats after cerebral ischemia/reperfusion. In this study we explored the protective mechanisms of caffeic acid against oxidative stress and ferroptosis in permanent cerebral ischemia. Ischemia stroke was induced on rats by permanent middle cerebral artery occlusion (pMCAO). Caffeic acid (0.4, 2, 10 mg·kg−1·d−1, i.g.) was administered to the rats for 3 consecutive days before or after the surgery. We showed that either pre-pMCAO or post-pMCAO administration of caffeic acid (2 mg·kg−1·d−1) effectively reduced the infarct volume and improved neurological outcome. The therapeutic time window could last to 2 h after pMCAO. We found that caffeic acid administration significantly reduced oxidative damage as well as neuroinflammation, and enhanced antioxidant capacity in pMCAO rat brain. We further demonstrated that caffeic acid down-regulated TFR1 and ACSL4, and up-regulated glutathione production through Nrf2 signaling pathway to resist ferroptosis in pMCAO rat brain and in oxygen glucose deprivation/reoxygenation (OGD/R)-treated SK-N-SH cells in vitro. Application of ML385, an Nrf2 inhibitor, blocked the neuroprotective effects of caffeic acid in both in vivo and in vitro models, evidenced by excessive accumulation of iron ions and inactivation of the ferroptosis defense system. In conclusion, caffeic acid inhibits oxidative stress-mediated neuronal death in pMCAO rat brain by regulating ferroptosis via Nrf2 signaling pathway. Caffeic acid might serve as a potential treatment to relieve brain injury after cerebral ischemia.

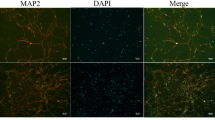
Caffeic acid significantly attenuated cerebral ischemic injury and resisted ferroptosis both in vivo and in vitro. The regulation of Nrf2 by caffeic acid initiated the transcription of downstream target genes, which were shown to be anti-inflammatory, antioxidative and antiferroptotic. The effects of caffeic acid on neuroinflammation and ferroptosis in cerebral ischemia were explored in a primary microglia-neuron coculture system. Caffeic acid played a role in reducing neuroinflammation and resisting ferroptosis through the Nrf2 signaling pathway, which further suggested that caffeic acid might be a potential therapeutic method for alleviating brain injury after cerebral ischemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Owolabi MO, Thrift AG, Mahal A, Ishida M, Martins S, Johnson WD, et al. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health. 2022;7:e74–e85.
Cui Y, Zhang Y, Zhao X, Shao L, Liu G, Sun C, et al. Acsl4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312–21.
Cohen JE, Itshayek E, Moskovici S, Gomori JM, Fraifeld S, Eichel R, et al. State-of-the-art reperfusion strategies for acute ischemic stroke. J Clin Neurosci. 2011;18:319–23.
Kelmanson IV, Shokhina AG, Kotova DA, Pochechuev MS, Ivanova AD, Kostyuk AI, et al. In vivo dynamics of acidosis and oxidative stress in the acute phase of an ischemic stroke in a rodent model. Redox Biol. 2021;48:102178.
Sharpe PC, Mulholland C, Trinick T. Ascorbate and malondialdehyde in stroke patients. Ir J Med Sci. 1994;163:488–91.
Domínguez C, Delgado P, Vilches A, Martín-Gallán P, Ribó M, Santamarina E, et al. Oxidative stress after thrombolysis-induced reperfusion in human stroke. Stroke. 2010;41:653–60.
Kimura-Ohba S, Yang Y. Oxidative DNA damage mediated by intranuclear mmp activity is associated with neuronal apoptosis in ischemic stroke. Oxid Med Cell Longev. 2016;2016:6927328.
Ichikawa H, Wang L, Konishi T. Prevention of cerebral oxidative injury by post-ischemic intravenous administration of shengmai san. Am J Chin Med. 2006;34:591–600.
Narne P, Pandey V, Phanithi PB. Role of nitric oxide and hydrogen sulfide in ischemic stroke and the emergent epigenetic underpinnings. Mol Neurobiol. 2019;56:1749–69.
Xu J, Wang A, Meng X, Yalkun G, Xu A, Gao Z, et al. Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: A phase iii, randomized, double-blind, comparative trial. Stroke. 2021;52:772–80.
Kobayashi S, Fukuma S, Ikenoue T, Fukuhara S, Kobayashi S. Effect of edaravone on neurological symptoms in real-world patients with acute ischemic stroke. Stroke. 2019;50:1805–11.
Chen T, Shi R, Suo Q, Wu S, Liu C, Huang S, et al. Progranulin released from microglial lysosomes reduces neuronal ferroptosis after cerebral ischemia in mice. J Cereb Blood Flow Metab. 2022;43:505–17.
Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19:405–14.
Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31.
Zhu L, Feng Z, Zhang J, Du L, Meng A. Microrna-27a regulates ferroptosis through slc7a11 to aggravate cerebral ischemia-reperfusion injury. Neurochem Res. 2023;48:1370–81.
Selim M. Treatment with the iron chelator, deferoxamine mesylate, alters serum markers of oxidative stress in stroke patients. Transl Stroke Res. 2010;1:35–9.
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–79.e1225.
Kaspar JW, Niture SK, Jaiswal AK. Nrf2:Inrf2 (keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–9.
Theodore M, Kawai Y, Yang J, Kleshchenko Y, Reddy SP, Villalta F, et al. Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J Biol Chem. 2008;283:8984–94.
Krajka-Kuzniak V, Paluszczak J, Baer-Dubowska W. The Nrf2-are signaling pathway: an update on its regulation and possible role in cancer prevention and treatment. Pharmacol Rep. 2017;69:393–402.
Pradeep H, Diya JB, Shashikumar S, Rajanikant GK. Oxidative stress-assassin behind the ischemic stroke. Folia Neuropathol. 2012;50:219–30.
Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–35.
Liu L, Vollmer MK, Fernandez VM, Dweik Y, Kim H, Dore S. Korean red ginseng pretreatment protects against long-term sensorimotor deficits after ischemic stroke likely through Nrf2. Front Cell Neurosci. 2018;12:74.
Duan J, Cui J, Yang Z, Guo C, Cao J, Xi M, et al. Neuroprotective effect of apelin 13 on ischemic stroke by activating AMPK/GSK-3beta/Nrf2 signaling. J Neuroinflammation. 2019;16:24.
Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2-are pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104.
Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114:237–46.
Agyeman AS, Chaerkady R, Shaw PG, Davidson NE, Visvanathan K, Pandey A, et al. Transcriptomic and proteomic profiling of keap1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res Treat. 2012;132:175–87.
Harada N, Kanayama M, Maruyama A, Yoshida A, Tazumi K, Hosoya T, et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem Biophys. 2011;508:101–9.
Campbell MR, Karaca M, Adamski KN, Chorley BN, Wang X, Bell DA. Novel hematopoietic target genes in the Nrf2-mediated transcriptional pathway. Oxid Med Cell Longev. 2013;2013:120305.
Khan FA, Maalik A, Murtaza G. Inhibitory mechanism against oxidative stress of caffeic acid. J Food Drug Anal. 2016;24:695–702.
Darendelioglu E. Caffeic acid suppresses HT-29 cell death induced by H2O2 via oxidative stress and apoptosis. Bratisl Lek Listy. 2020;121:805–11.
Jiang RW, Lau KM, Hon PM, Mak TC, Woo KS, Fung KP. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem. 2005;12:237–46.
Yang L, Tao Y, Luo L, Zhang Y, Wang X, Meng X. Dengzhan xixin injection derived from a traditional Chinese herb erigeron breviscapus ameliorates cerebral ischemia/reperfusion injury in rats via modulation of mitophagy and mitochondrial apoptosis. J Ethnopharmacol. 2022;288:114988.
Luo L, Wu S, Chen R, Rao H, Peng W, Su W. The study of neuroprotective effects and underlying mechanism of naoshuantong capsule on ischemia stroke mice. Chin Med. 2020;15:119.
Mu X, Xu X, Guo X, Yang P, Du J, Mi N, et al. Identification and characterization of chemical constituents in dengzhan shengmai capsule and their metabolites in rat plasma by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2019;1108:54–64.
Zhou Y, Fang SH, Ye YL, Chu LS, Zhang WP, Wang ML, et al. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol Sin. 2006;27:1103–10.
Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the united states: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–5.
Gilgun-Sherki Y, Rosenbaum Z, Melamed E, Offen D. Antioxidant therapy in acute central nervous system injury: current state. Pharmacol Rev. 2002;54:271–84.
Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J Cereb Blood Flow Metab. 2014;34:1573–84.
Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–72.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91.
Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008;153:S396–405.
Engel O, Kolodziej S, Dirnagl U, Prinz V. Modeling stroke in mice - middle cerebral artery occlusion with the filament model. J Vis Exp. 2011;6:2423.
Feng H, Hu L, Zhu H, Tao L, Wu L, Zhao Q, et al. Repurposing antimycotic ciclopirox olamine as a promising anti-ischemic stroke agent. Acta Pharm Sin B. 2020;10:434–46.
Hu Q, Zuo T, Deng L, Chen S, Yu W, Liu S, et al. caryophyllene suppresses ferroptosis induced by cerebral ischemia reperfusion via activation of the Nrf2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine. 2022;102:154112.
Peng Y, Shao-Feng XU, Wang L, Feng YP, Wang XL. Effects of chiral NBP on cerebral infarct volume due to transient focal cerebral ischemia. Chin New Drugs J. 2005;14:420–3.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–6.
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11.
Xue Q, Yan D, Chen X, Li X, Kang R, Klionsky DJ, et al. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy. 2023;19:1982–96.
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–99.
Zhu G, Wang X, Chen L, Lenahan C, Fu Z, Fang Y, et al. Crosstalk between the oxidative stress and glia cells after stroke: from mechanism to therapies. Front Immunol. 2022;13:852416.
Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20:795–820.
Xu R, Wang L, Sun L, Dong J. Neuroprotective effect of magnesium supplementation on cerebral ischemic diseases. Life Sci. 2021;272:119257.
Cheng Y, Song Y, Chen H, Li Q, Gao Y, Lu G, et al. Ferroptosis mediated by lipid reactive oxygen species: a possible causal link of neuroinflammation to neurological disorders. Oxid Med Cell Longev. 2021;2021:5005136.
Kashiwada Y, Nishizawa M, Yamagishi T, Tanaka T, Nonaka G, Cosentino LM, et al. Anti-aids agents, 18. Sodium and potassium salts of caffeic acid tetramers from arnebia euchroma as anti-hiv agents. J Nat Prod. 1995;58:392–400.
Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–70.
Kępa M, Miklasińska-Majdanik M, Wojtyczka RD, Idzik D, Korzeniowski K, Smoleń-Dzirba J, et al. Antimicrobial potential of caffeic acid against staphylococcus aureus clinical strains. Biomed Res Int. 2018;2018:7413504.
Khan F, Bamunuarachchi NI, Tabassum N, Kim YM. Caffeic acid and its derivatives: antimicrobial drugs toward microbial pathogens. J Agric Food Chem. 2021;69:2979–3004.
Koga M, Nakagawa S, Kato A, Kusumi I. Caffeic acid reduces oxidative stress and microglial activation in the mouse hippocampus. Tissue Cell. 2019;60:14–20.
Muhammad Abdul Kadar NN, Ahmad F, Teoh SL, Yahaya MF. Caffeic acid on metabolic syndrome: a review. Molecules. 2021;26:4777.
He F, Ru X. Wen T. Nrf2, a transcription factor for stress response and beyond. Int J Mol Sci. 2020;21:4777.
Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–41.
Rojo AI, Rada P, Egea J, Rosa AO, López MG, Cuadrado A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol Cell Neurosci. 2008;39:125–32.
Ali T, Kim T, Rehman SU, Khan MS, Amin FU, Khan M, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of alzheimer’s disease. Mol Neurobiol. 2018;55:6076–93.
Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830:3143–53.
Hribljan V, Lisjak D, Petrović DJ, Mitrečić D. Necroptosis is one of the modalities of cell death accompanying ischemic brain stroke: from pathogenesis to therapeutic possibilities. Croat Med J. 2019;60:121–6.
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98:813–80.
Dodson M, Castro-Portuguez R, Zhang DD. Nrf2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107.
Hirschhorn T, Stockwell BR. The development of the concept of ferroptosis. Free Radic Biol Med. 2019;133:130–43.
Lu Y, Yang Q, Su Y, Ji Y, Li G, Yang X, et al. Mycn mediates tfrc-dependent ferroptosis and reveals vulnerabilities in neuroblastoma. Cell Death Dis. 2021;12:511.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. Acsl4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98.
Shimada K, Hayano M, Pagano NC, Stockwell BR. Cell-line selectivity improves the predictive power of pharmacogenomic analyses and helps identify nadph as biomarker for ferroptosis sensitivity. Cell Chem Biol. 2016;23:225–35.
Klett EL, Chen S, Yechoor A, Lih FB, Coleman RA. Long-chain acyl-coa synthetase isoforms differ in preferences for eicosanoid species and long-chain fatty acids. J Lipid Res. 2017;58:884–94.
Klett EL, Chen S, Edin ML, Li LO, Ilkayeva O, Zeldin DC, et al. Diminished acyl-coa synthetase isoform 4 activity in ins 832/13 cells reduces cellular epoxyeicosatrienoic acid levels and results in impaired glucose-stimulated insulin secretion. J Biol Chem. 2013;288:21618–29.
Kang MJ, Fujino T, Sasano H, Minekura H, Yabuki N, Nagura H, et al. A novel arachidonate-preferring Acyl-Coa synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc Natl Acad Sci USA. 1997;94:2880–4.
Liao S, Wu J, Liu R, Wang S, Luo J, Yang Y, et al. A novel compound DBZ ameliorates neuroinflammation in LPS-stimulated microglia and ischemic stroke rats: role of AKT(ser473)/GSK3β(ser9)-mediated Nrf2 activation. Redox Biol. 2020;36:101644.
da Silveira M, Luz DACCS, da Silva CCS, Prediger RDS, Martins MD, Martins MAT, et al. Propolis: a useful agent on psychiatric and neurological disorders? A focus on cape and pinocembrin components. Med Res Rev. 2021;41:1195–215.
Guruswamy R, ElAli A. Complex roles of microglial cells in ischemic stroke pathobiology: new insights and future directions. Int J Mol Sci. 2017;18:496.
Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068.
Zhang Z, Lv M, Zhou X, Cui Y. Roles of peripheral immune cells in the recovery of neurological function after ischemic stroke. Front Cell Neurosci. 2022;16:1013905.
Kumar A, Chen SH, Kadiiska MB, Hong JS, Zielonka J, Kalyanaraman B, et al. Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells. Free Radic Biol Med. 2014;73:51–9.
Yang JQ, Zhou QX, Liu BZ, He BC. Protection of mouse brain from aluminum-induced damage by caffeic acid. CNS Neurosci Ther. 2008;14:10–6.
Adamek A, Jung S, Dienesch C, Laser M, Ertl G, Bauersachs J, et al. Role of 5-lipoxygenase in myocardial ischemia-reperfusion injury in mice. Eur J Pharmacol. 2007;571:51–54.
Kitagawa K, Matsumoto M, Hori M. Cerebral ischemia in 5-lipoxygenase knockout mice. Brain Res. 2004;1004:198–202.
Patel NS, Cuzzocrea S, Chatterjee PK, Di Paola R, Sautebin L, Britti D, et al. Reduction of renal ischemia-reperfusion injury in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. Mol Pharmacol. 2004;66:220–7.
Lisovyy OO, Dosenko VE, Nagibin VS, Tumanovska LV, Korol MO, Surova OV, et al. Cardioprotective effect of 5-lipoxygenase gene (ALOX5) silencing in ischemia-reperfusion. Acta Biochim Pol. 2009;56:687–94.
Shi KN, Li PB, Su HX, Gao J, Li HH. MK-886 protects against cardiac ischaemia/reperfusion injury by activating proteasome-keap1-Nrf2 signalling. Redox Biol. 2023;62:102706.
Acknowledgements
This project was supported by grants from the National Key R&D Program of China (No. 2019YFC1708901), the National Natural Science Foundation of China (No. 82073835), and the CAMS Innovation Fund for Medical Sciences (No. 2021-I2M-1-028 and 2022-I2M-2-002).
Author information
Authors and Affiliations
Contributions
XNL: Performing the experiments, Investigation, Methodology, Validation, Formal analysis, Writing the original draft; NYS: Performing the experiments, Investigation, Methodology, Validation, Formal analysis, Writing the original draft; YYK: Performing the experiments, Methodology; NS: Performing the experiments, Methodology; JQL: Performing the experiments, Methodology; JST: Performing the experiments, Methodology; LW: Methodology, Supervision; JLZ: Conceptualization, Funding acquisition, Project administration, Supervision, Validation; YP: Conceptualization, Funding acquisition, Project administration, Supervision, Validation and Writing-review. All authors have read and approved the final manuscript. All data were generated in-house and no paper mill was used. All authors agree to be accountable for all aspects of the work, ensuring its integrity and accuracy.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Xn., Shang, Ny., Kang, Yy. et al. Caffeic acid alleviates cerebral ischemic injury in rats by resisting ferroptosis via Nrf2 signaling pathway. Acta Pharmacol Sin 45, 248–267 (2024). https://doi.org/10.1038/s41401-023-01177-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01177-5