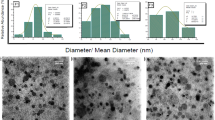
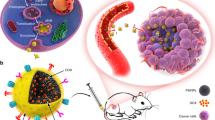
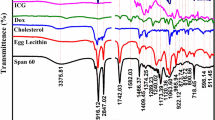
Abstract
Higher drug loading employed in nanoscale delivery platforms is a goal that researchers have long sought after. But such viewpoint remains controversial because the impacts that nanocarriers bring about on bodies have been seriously overlooked. In the present study we investigated the effects of drug loading on the in vivo performance of PEGylated liposomal doxorubicin (PLD). We prepared PLDs with two different drug loading rates: high drug loading rate, H-Dox, 12.9% w/w Dox/HSPC; low drug loading rate, L-Dox, 2.4% w/w Dox/HSPC (L-Dox had about 5 folds drug carriers of H-Dox at the same Dox dose). The pharmaceutical properties and biological effects of H-Dox and L-Dox were compared in mice, rats or 4T1 subcutaneous tumor-bearing mice. We showed that the lowering of doxorubicin loading did not cause substantial shifts to the pharmaceutical properties of PLDs such as in vitro and in vivo stability (stable), anti-tumor effect (equivalent effective), as well as tissue and cellular distribution. Moreover, it was even more beneficial for mitigating the undesired biological effects caused by PLDs, through prolonging blood circulation and alleviating cutaneous accumulation in the presence of pre-existing anti-PEG Abs due to less opsonins (e.g. IgM and C3) deposition on per particle. Our results warn that the effects of drug loading would be much more convoluted than expected due to the complex intermediation between nanocarriers and bodies, urging independent investigation for each individual delivery platform to facilitate clinical translation and application.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and the supplementary information. All other data are available from the authors upon reasonable request.
References
Langer R. Drug delivery and targeting. Nature. 1998;392:5–10.
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71.
Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–8.
Gregoriadis G, Wills EJ, Swain CP, Tavill AS. Drug-carrier potential of liposomes in cancer chemotherapy. Lancet. 1974;1:1313–6.
Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853–8.
Duggan ST, Keating GM. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 2011;71:2531–58.
Barenholz Y. Doxil®–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–34.
Akhtar N, Mohammed SA, Singh V, Abdellatif AA, Mohammad HA, Ahad A, et al. Liposome-based drug delivery of various anticancer agents of synthetic and natural product origin: a patent overview. Pharm Pat Anal. 2020;9:87–116.
Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1:297–315.
Maiti P. Drug-delivery vehicles and their efficiency toward cancer treatment. Nanomed (Lond). 2020;15:1637–40.
Kita K, Dittrich C. Drug delivery vehicles with improved encapsulation efficiency: taking advantage of specific drug–carrier interactions. Expert Opin Drug Deliv. 2011;8:329–42.
Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–75.
Gerecke C, Edlich A, Giulbudagian M, Schumacher F, Zhang N, Said A, et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology. 2017;11:267–77.
Park H, Park K. Biocompatibility issues of implantable drug delivery systems. Pharmacol Res. 1996;13:1770–6.
Tang Y, Wang X, Li J, Nie Y, Liao G, Yu Y, et al. Overcoming the reticuloendothelial system barrier to drug delivery with a “Don’t-Eat-Us” strategy. ACS Nano. 2019;13:13015–26.
Liu X, Tang I, Wainberg ZA, Meng H. Safety considerations of cancer nanomedicine—a key step toward translation. Small. 2020;16:e2000673.
Brand W, Noorlander CW, Giannakou C, De Jong WH, Kooi MW, Park MV, et al. Nanomedicinal products: a survey on specific toxicity and side effects. Int J Nanomed. 2017;12:6107–29.
Caracciolo G. Liposome-protein corona in a physiological environment: challenges and opportunities for targeted delivery of nanomedicines. Nanomedicine. 2015;11:543–57.
Zahednezhad F, Saadat M, Valizadeh H, Zakeri-Milani P, Baradaran B. Liposome and immune system interplay: challenges and potentials. J Control Release. 2019;305:194–209.
Onishchenko N, Tretiakova D, Vodovozova E. Spotlight on the protein corona of liposomes. Acta Biomater. 2021;134:57–78.
Yang K, Reker-Smit C, Stuart MCA, Salvati A. Effects of protein source on liposome uptake by cells: Corona composition and impact of the excess free proteins. Adv Health Mater. 2021;10:e2100370.
Moghimi SM, Hamad I. Liposome-mediated triggering of complement cascade. J Liposome Res. 2008;18:195–209.
Yan X, Scherphof GL, Kamps JA. Liposome opsonization. J Liposome Res. 2005;15:109–39.
Clogston JD. The importance of nanoparticle physicochemical characterization for immunology research: what we learned and what we still need to understand. Adv Drug Deliv Rev. 2021;176:113897.
Luo R, Li Y, He M, Zhang H, Yuan H, Johnson M, et al. Distinct biodistribution of doxorubicin and the altered dispositions mediated by different liposomal formulations. Int J Pharm. 2017;519:1–10.
Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXILI/CAELYXI) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2001;37:870–7.
Shafei A, El-Bakly W, Sobhy A, Wagdy O, Reda A, Aboelenin O, et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed Pharmacother. 2017;95:1209–18.
DOXIL® (doxorubicin HCl liposome injection) for intravenous infusion, https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050718s029lbl.pdf (2007).
Lotem M, Hubert A, Lyass O, Goldenhersh MA, Ingber A, Peretz T, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475–80.
Li Y, Lofchy L, Wang G, Gaikwad H, Fujita M, Simberg D. PEGylated liposomes accumulate in the areas relevant to skin toxicities via passive extravasation across “Leaky” Endothelium. ACS Nano. 2022;16:6349–58.
Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151:201–15.
Chu Y, Tang W, Zhang Z, Li C, Qian J, Wei X, et al. Deciphering protein Corona by scFv-based affinity chromatography. Nano Lett. 2021;21:2124–31.
Zhang Z, Chu Y, Li C, Tang W, Qian J, Wei X, et al. Anti-PEG scFv corona ameliorates accelerated blood clearance phenomenon of PEGylated nanomedicines. J Control Release. 2021;330:493–501.
Tang W, Zhang Z, Li C, Chu Y, Qian J, Ying T, et al. Facile separation of PEGylated liposomes enabled by anti-PEG scFv. Nano Lett. 2021;21:10107–13.
Choi WG, Kim DK, Shin Y, Park R, Cho YY, Lee JY, et al. Liquid chromatography-tandem mass spectrometry for the simultaneous determination of doxorubicin and its metabolites doxorubicinol, doxorubicinone, doxorubicinolone, and 7-deoxydoxorubicinone in mouse plasma. Molecules. 2020;25:1254.
Wei X, Shamrakov D, Nudelman S, Peretz-Damari S, Nativ-Roth E, Regev O, et al. Cardinal role of intraliposome doxorubicin-sulfate nanorod crystal in doxil properties and performance. ACS Omega. 2018;3:2508–17.
Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51.
Chen BM, Su YC, Chang CJ, Burnouf PA, Chuang KH, Chen CH, et al. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem. 2016;88:10661–6.
Povsic TJ, Lawrence MG, Lincoff AM, Mehran R, Rusconi CP, Zelenkofske SL, et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol. 2016;138:1712–5.
Wang H, Ding T, Guan J, Liu X, Wang J, Jin P, et al. Interrogation of Folic Acid-functionalized nanomedicines: the regulatory roles of plasma proteins reexamined. ACS Nano. 2020;14:14779–89.
Ding T, Guan J, Wang M, Long Q, Liu X, Qian J, et al. Natural IgM dominates in vivo performance of liposomes. J Control Release. 2020;319:371–81.
Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710–24.
Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:655–77.
Kozma GT, Shimizu T, Ishida T, Szebeni J. Anti-PEG antibodies: properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv Drug Deliv Rev. 2020;154-155:163–75.
Tan LA, Yu B, Sim FC, Kishore U, Sim RB. Complement activation by phospholipids: the interplay of factor H and C1q. Protein Cell. 2010;1:1033–49.
Kawanishi M, Hashimoto Y, Shimizu T, Sagawa I, Ishida T, Kiwada H. Comprehensive analysis of PEGylated liposome-associated proteins relating to the accelerated blood clearance phenomenon by combination with shotgun analysis and conventional methods. Biotechnol Appl Biochem. 2015;62:547–55.
Sun X, Yan X, Jacobson O, Sun W, Wang Z, Tong X, et al. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics. 2017;7:319–28.
Tavares AJ, Poon W, Zhang YN, Dai Q, Besla R, Ding D, et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci USA. 2017;114:E10871–E80.
Blandino R, Baumgarth N. Secreted IgM: new tricks for an old molecule. J Leukoc Biol. 2019;106:1021–34.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520.
Hadjidemetriou M, McAdam S, Garner G, Thackeray C, Knight D, Smith D, et al. The human in vivo biomolecule Corona onto PEGylated liposomes: a proof-of-concept clinical study. Adv Mater. 2019;31:e1803335.
Ju Y, Kelly HG, Dagley LF, Reynaldi A, Schlub TE, Spall SK, et al. Person-specific biomolecular Coronas modulate nanoparticle interactions with immune cells in human blood. ACS Nano. 2020;14:15723–37.
Vu VP, Gifford GB, Chen F, Benasutti H, Wang G, Groman EV, et al. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat Nanotechnol. 2019;14:260–8.
Guan J, Shen Q, Zhang Z, Jiang Z, Yang Y, Lou M, et al. Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nat Commun. 2018;9:2982.
Neun B, Barenholz Y, Szebeni J, Dobrovolskaia M. Understanding the role of anti-PEG antibodies in the complement activation by Doxil in vitro. Molecules. 2018;23:1700.
Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release. 2006;115:251–8.
Shimizu T, Ishida T, Kiwada H. Transport of PEGylated liposomes from the splenic marginal zone to the follicle in the induction phase of the accelerated blood clearance phenomenon. Immunobiology. 2013;218:725–32.
Han Y, Liu Q, Hou J, Gu Y, Zhang Y, Chen Z, et al. Tumor-induced generation of splenic Erythroblast-like Ter-cells promotes tumor progression. Cell. 2018;173:634–48.
Tanaka K, Koga Y, Taniguchi K, Kamikaseda K, Nihashi Y, Nomoto K. T-cell recruitment from the thymus to the spleen in tumor-bearing mice. II. Functional characteristics of recruited T -cells. J Natl Cancer Inst. 1986;77:733–8.
Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol. 2019;4:eaau6085.
Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16.
Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306–21.
Wculek SK, Dunphy G, Heras-Murillo I, Mastrangelo A, Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol. 2022;19:384–408.
Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev. 2011;37:63–74.
Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166.
Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1482–92.
Ouyang B, Poon W, Zhang YN, Lin ZP, Kingston BR, Tavares AJ, et al. The dose threshold for nanoparticle tumour delivery. Nat Mater. 2020;19:1362–71.
Proffitt RT, Williams LE, Presant CA, Tin GW, Uliana JA, Gamble RC, et al. Liposoinal blockade of the reticuloendothelial system: improved tumor imaging with small unilamellar vesicles. Science. 1983;220:502–5.
Liu T, Choi H, Zhou R, Chen IW. RES blockade: a strategy for boosting efficiency of nanoparticle drug. Nano Today. 2015;10:11–21.
Szebeni J, Baranyi L, Savay S, Milosevits J, Bunger R, Laverman P, et al. Role of complement activation in hypersensitivity reactions to doxil and hynic PEG liposomes: experimental and clinical studies. J Liposome Res. 2002;12:165–72.
Estape Senti M, de Jongh CA, Dijkxhoorn K, Verhoef JJF, Szebeni J, Storm G, et al. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J Control Release. 2022;341:475–86.
Chen E, Chen BM, Su YC, Chang YC, Cheng TL, Barenholz Y, et al. Premature drug release from polyethylene glycol (PEG)-coated liposomal doxorubicin via formation of the membrane attack complex. ACS Nano. 2020;14:7808–22.
Gabizon A. Pharmacokinetics of pegylated liposomal doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42:419–36.
Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20–37.
Shi D, Beasock D, Fessler A, Szebeni J, Ljubimova JY, Afonin KA, et al. To PEGylate or not to PEGylate: immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv Drug Deliv Rev. 2022;180:114079.
Liposome Drug Products: Chemistry, manufacturing, and controls; human pharmacokinetics and bioavailability; and labeling documentation, https://www.fda.gov/media/70837/download (2018).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (82273866, 82125035, 81973245) and Shanghai Education Commission Major Project (2021-01-07-00-07-E00081).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, Yf., Wu, Ec., Lin, Sq. et al. Reexamining the effects of drug loading on the in vivo performance of PEGylated liposomal doxorubicin. Acta Pharmacol Sin 45, 646–659 (2024). https://doi.org/10.1038/s41401-023-01169-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01169-5