Abstract
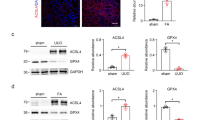
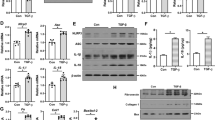
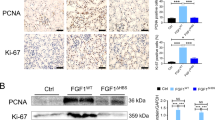
Kidney fibrosis is the hallmark of chronic kidney disease (CKD) progression, whereas no effective anti-fibrotic therapies exist. Recent evidence has shown that tubular ferroptosis contributes to the pathogenesis of CKD with persistent proinflammatory and profibrotic responses. We previously reported that natural flavonol fisetin alleviated septic acute kidney injury and protected against hyperuricemic nephropathy in mice. In this study, we investigated the therapeutic effects of fisetin against fibrotic kidney disease and the underlying mechanisms. We established adenine diet-induced and unilateral ureteral obstruction (UUO)-induced CKD models in adult male mice. The two types of mice were administered fisetin (50 or 100 mg·kg−1·d−1, i.g.) for 3 weeks or 7 days, respectively. At the end of the experiments, the mice were euthanized, and blood and kidneys were gathered for analyzes. We showed that fisetin administration significantly ameliorated tubular injury, inflammation, and tubulointerstitial fibrosis in the two types of CKD mice. In mouse renal tubular epithelial (TCMK-1) cells, treatment with fisetin (20 μM) significantly suppressed adenine- or TGF-β1-induced inflammatory responses and fibrogenesis, and improved cell viability. By quantitative real-time PCR analysis of ferroptosis-related genes, we demonstrated that fisetin treatment inhibited ferroptosis in the kidneys of CKD mice as well as in injured TCMK-1 cells, as evidenced by decreased ACSL4, COX2, and HMGB1, and increased GPX4. Fisetin treatment effectively restored ultrastructural abnormalities of mitochondrial morphology and restored the elevated iron, the reduced GSH and GSH/GSSG as well as the increased lipid peroxide MDA in the kidneys of CKD mice. Notably, abnormally high expression of the ferroptosis key marker ACSL4 was verified in the renal tubules of CKD patients (IgAN, MN, FSGS, LN, and DN) as well as adenine- or UUO-induced CKD mice, and in injured TCMK-1 cells. In adenine- and TGF-β1-treated TCMK-1 cells, ACSL4 knockdown inhibited tubular ferroptosis, while ACSL4 overexpression blocked the anti-ferroptotic effect of fisetin and reversed the cytoprotective, anti-inflammatory, and anti-fibrotic effects of fisetin. In summary, we reveal a novel aspect of the nephroprotective effect of fisetin, i.e. inhibiting ACSL4-mediated tubular ferroptosis against fibrotic kidney diseases.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Sundström J, Bodegard J, Bollmann A, Vervloet MG, Mark PB, Karasik A, et al. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: the CaReMe CKD study. Lancet Reg Health Eur. 2022;20:100438.
Collaboration. GCKD. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–33.
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392:2052–90.
Yuan Q, Tang B, Zhang C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct Target Ther. 2022;7:182.
Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–7.
Huang R, Fu P, Ma L. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct Target Ther. 2023;8:129.
Yu SM, Bonventre JV. Acute kidney injury and maladaptive tubular repair leading to renal fibrosis. Curr Opin Nephrol Hypertens. 2020;29:310–8.
Guo JK, Cantley LG. Cellular maintenance and repair of the kidney. Annu Rev Physiol. 2010;72:357–76.
Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–96.
Li H, Dixon EE, Wu H, Humphreys BD. Comprehensive single-cell transcriptional profiling defines shared and unique epithelial injury responses during kidney fibrosis. Cell Metab. 2022;34:1977–1998.e9.
Liu BC, Tang TT, Lv LL, Lan HY. Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 2018;93:568–79.
Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–21.
Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25.
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88.
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8.
Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A. Ferroptosis and necroptosis in the kidney. Cell Chem Biol. 2020;27:448–62.
Wang Y, Zhang M, Bi R, Su Y, Quan F, Lin Y, et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022;51:102262.
Zhou L, Xue X, Hou Q, Dai C. Targeting ferroptosis attenuates interstitial inflammation and kidney fibrosis. Kidney Dis. 2022;8:57–71.
Wang J, Wang Y, Liu Y, Cai X, Huang X, Fu W, et al. Ferroptosis, a new target for treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced CKD rat model. Cell Death Discov. 2022;8:127.
Zhang B, Chen X, Ru F, Gan Y, Li B, Xia W, et al. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis. 2021;12:843.
Li X, Zou Y, Xing J, Fu YY, Wang KY, Wan PZ, et al. Pretreatment with roxadustat (FG-4592) attenuates folic acid-induced kidney injury through antiferroptosis via Akt/GSK-3β/Nrf2 pathway. Oxid Med Cell Longev. 2020;2020:6286984.
Kim S, Kang SW, Joo J, Han SH, Shin H, Nam BY, et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12:160.
Li S, Zheng L, Zhang J, Liu X, Wu Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435–49.
Khan MA, Nag P, Grivei A, Giuliani KTK, Wang X, Diwan V, et al. Adenine overload induces ferroptosis in human primary proximal tubular epithelial cells. Cell Death Dis. 2022;13:104.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803.
Rengarajan T, Yaacob NS. The flavonoid fisetin as an anticancer agent targeting the growth signaling pathways. Eur J Pharmacol. 2016;789:8–16.
Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal. 2013;19:151–62.
Touil YS, Auzeil N, Boulinguez F, Saighi H, Regazzetti A, Scherman D, et al. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochem Pharmacol. 2011;82:1731–9.
Ren Q, Tao S, Guo F, Wang B, Yang L, Ma L, et al. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomedicine. 2021;87:153552.
Yang J, Li Q, Henning SM, Zhong J, Hsu M, Lee R, et al. Effects of prebiotic fiber xylooligosaccharide in adenine-induced nephropathy in mice. Mol Nutr Food Res. 2018;62:e1800014.
Buchtler S, Grill A, Hofmarksrichter S, Stöckert P, Schiechl-Brachner G, Rodriguez Gomez M, et al. Cellular origin and functional relevance of collagen I production in the kidney. J Am Soc Nephrol. 2018;29:1859–73.
Schley G, Klanke B, Kalucka J, Schatz V, Daniel C, Mayer M, et al. Mononuclear phagocytes orchestrate prolyl hydroxylase inhibition-mediated renoprotection in chronic tubulointerstitial nephritis. Kidney Int. 2019;96:378–96.
Zheng M, Hu Z, Wang Y, Wang C, Zhong C, Cui W, et al. Zhen Wu decoction represses renal fibrosis by invigorating tubular NRF2 and TFAM to fuel mitochondrial bioenergetics. Phytomedicine. 2023;108:154495.
Feng Y, Guo F, Xia Z, Liu J, Mai H, Liang Y, et al. Inhibition of fatty acid-binding protein 4 attenuated kidney fibrosis by mediating macrophage-to-myofibroblast transition. Front Immunol. 2020;11:566535.
Wang B, Xu J, Ren Q, Cheng L, Guo F, Liang Y, et al. Fatty acid-binding protein 4 is a therapeutic target for septic acute kidney injury by regulating inflammatory response and cell apoptosis. Cell Death Dis. 2022;13:333.
Liu N, Wang L, Yang T, Xiong C, Xu L, Shi Y, et al. EGF receptor inhibition alleviates hyperuricemic nephropathy. J Am Soc Nephrol. 2015;26:2716–29.
Zitka O, Skalickova S, Gumulec J, Masarik M, Adam V, Hubalek J, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol Lett. 2012;4:1247–53.
Pan J, Shi M, Guo F, Ma L, Fu P. Pharmacologic inhibiting STAT3 delays the progression of kidney fibrosis in hyperuricemia-induced chronic kidney disease. Life Sci. 2021;285:119946.
Zhou N, Bao J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database (Oxford). 2020;2020:baaa021.
Wu H, Kirita Y, Donnelly EL, Humphreys BD. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J Am Soc Nephrol. 2019;30:23–32.
Kirita Y, Wu H, Uchimura K, Wilson PC, Humphreys BD. Cell profiling of mouse acute kidney injury reveals conserved cellular responses to injury. Proc Natl Acad Sci USA. 2020;117:15874–83.
Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83.
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7:193.
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, et al. The emerging role of ferroptosis in inflammation. Biomed Pharmacother. 2020;127:110108.
Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–43.
Gan B. ACSL4, PUFA, and ferroptosis: new arsenal in anti-tumor immunity. Signal Transduct Target Ther. 2022;7:128.
Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother. 2020;122:109772.
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, et al. Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther. 2021;6:49.
Zhang Y, Mou Y, Zhang J, Suo C, Zhou H, Gu M, et al. Therapeutic implications of ferroptosis in renal fibrosis. Front Mol Biosci. 2022;9:890766.
Ikeda Y, Ozono I, Tajima S, Imao M, Horinouchi Y, Izawa-Ishizawa Y, et al. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS One. 2014;9:e89355.
Wu T, Wang X, Chen M, Zhang X, Zhang J, Cheng J, et al. Respiratory exposure to graphene quantum dots causes fibrotic effects on lung, liver and kidney of mice. Food Chem Toxicol. 2022;163:112971.
Tao WH, Shan XS, Zhang JX, Liu HY, Wang BY, Wei X, et al. Dexmedetomidine attenuates ferroptosis-mediated renal ischemia/reperfusion injury and inflammation by inhibiting ACSL4 via α2-AR. Front Pharmacol. 2022;13:782466.
Acknowledgements
This current work was supported by the Science/Technology Project of Sichuan Province (2021YFQ0027 and 2022YFS0589) and the Health Commission of Sichuan Province (20PJ048). The graphical abstract was created with a free version of Biorender.com under agreement number EL24NF7RFD.
Author information
Authors and Affiliations
Contributions
LM and BW designed experiments. BW, LNY, and YL performed experiments. BW, LNY, LTY, FG, and LM analyzed the data. BW, LNY, and LM wrote the draft of the manuscript and edited it. All authors have read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, B., Yang, Ln., Yang, Lt. et al. Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis. Acta Pharmacol Sin 45, 150–165 (2024). https://doi.org/10.1038/s41401-023-01156-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01156-w