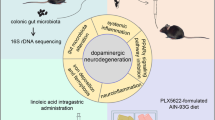
Abstract
The gut-brain axis plays a vital role in Parkinson’s disease (PD). The mechanisms of gut-brain transmission mainly focus on α-synuclein deposition, intestinal inflammation and microbiota function. A few studies have shown the trigger of PD pathology in the gut. α-Synuclein is highly conserved in food products, which was able to form β-folded aggregates and to infect the intestinal mucosa. In this study we investigated whether α-synuclein-preformed fibril (PFF) exposure could modulate the intestinal environment and induce rodent models replicating PD pathology. We first showed that PFF could be internalized into co-cultured Caco-2/HT29/Raji b cells in vitro. Furthermore, we demonstrated that PFF perfusion caused the intestinal inflammation and activation of enteric glial cells in an ex vivo intestinal organ culture and in an in vivo intestinal mouse coloclysis model. Moreover, we found that PFF exposure through regular coloclysis induced PD pathology in wild-type (WT) and A53T α-synuclein transgenic mice with various phenotypes. Particularly in A53T mice, PFF induced significant behavioral disorders, intestinal inflammation, α-synuclein deposition, microbiota dysbiosis, glial activation as well as degeneration of dopaminergic neurons in the substantia nigra. In WT mice, however, the PFF induced only mild behavioral abnormalities, intestinal inflammation, α-synuclein deposition, and glial activation, without significant changes in microbiota and dopaminergic neurons. Our results reveal the possibility of α-synuclein aggregates binding to the intestinal mucosa and modeling PD in mice. This study may shed light on the investigation and early intervention of the gut-origin hypothesis in neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912.
Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol. 1991;50:743–55.
Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96.
Vidailhet M. Parkinson disease—symptoms and treatments. Nat Rev Neurol. 2011;7:70–2.
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–34.
Dogra N, Mani RJ, Katare DP. The gut-brain axis: two ways signaling in Parkinson’s disease. Cell Mol Neurobiol. 2022;42:315–32.
Challis C, Hori A, Sampson TR, Yoo BB, Challis RC, Hamilton AM, et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci. 2020;23:327–36.
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128:805–20.
Kim S, Kwon SH, Kam TI, Panicker N, Karuppagounder SS, Lee S, et al. Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–.e7.
Park S, Kim J, Chun J, Han K, Soh H, Kang EA, et al. Patients with inflammatory bowel disease are at an increased risk of Parkinson’s disease: a South Korean nationwide population-based study. J Clin Med. 2019;8:1191.
Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, et al. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One. 2010;5:e8762.
Kishimoto Y, Zhu W, Hosoda W, Sen JM, Mattson MP. Chronic mild gut inflammation accelerates brain neuropathology and motor dysfunction in α-synuclein mutant mice. Neuromol Med. 2019;21:239–49.
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–.e12.
Pellegrini C, D’Antongiovanni V, Miraglia F, Rota L, Benvenuti L, Di Salvo C, et al. Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology. NPJ Parkinsons Dis. 2022;8:9.
Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3.
Oppong GO, Rapsinski GJ, Tursi SA, Biesecker SG, Klein-Szanto AJP, Goulian M, et al. Biofilm-associated bacterial amyloids dampen inflammation in the gut: oral treatment with curli fibres reduces the severity of hapten-induced colitis in mice. NPJ Biofilms Microbiomes. 2015;1:15019.
Sampson TR, Challis C, Jain N, Moiseyenko A, Ladinsky MS, Shastri GG, et al. A gut bacterial amyloid promotes a-synuclein aggregation and motor impairment in mice. Elife. 2020;9:1–19.
Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, et al. Prion-like spreading of pathological α-synuclein in brain. Brain. 2013;136:1128–38.
Paslawski W, Andreasen M, Nielsen SB, Lorenzen N, Thomsen K, Kaspersen JD, et al. High stability and cooperative unfolding of α-synuclein oligomers. Biochemistry. 2014;53:6252–63.
Marczynski M, Rickert CA, Semerdzhiev SA, van Dijk WR, Segers-Nolten IMJ, Claessens MMAE, et al. α-Synuclein penetrates mucin hydrogels despite its mucoadhesive properties. Biomacromolecules. 2019;20:4332–44.
Lohmann S, Bernis ME, Tachu BJ, Ziemski A, Grigoletto J, Tamgüney G. Oral and intravenous transmission of α-synuclein fibrils to mice. Acta Neuropathol. 2019;138:515–33.
Killinger BA, Labrie V. Vertebrate food products as a potential source of prion-like α-synuclein. NPJ Parkinsons Dis. 2017;3:33.
Lerner A. The intestinal luminal sources of α-synuclein: a gastroenterologist perspective. Nutr Rev. 2022;80:282–93.
Hu H, Wang Q, Du J, Liu Z, Ding Y, Xue H, et al. Aha1 exhibits distinctive dynamics behavior and chaperone-like activity. Molecules. 2021;26:1943.
Barlow-Anacker AJ, Erickson CS, Epstein ML, Gosain A. Immunostaining to visualize murine enteric nervous system development. J Visual Exp. 2015;29:e52716.
Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VMY. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–33.
Yissachar N, Zhou Y, Ung L, Lai NY, Mohan JF, Ehrlicher A, et al. An intestinal organ culture system uncovers a role for the nervous system in microbe-immune crosstalk. Cell. 2017;168:1135–e12.
Wu Q, Yang X, Zhang Y, Zhang L, Feng L. Chronic mild stress accelerates the progression of Parkinson’s disease in A53T α-synuclein transgenic mice. Exp Neurol. 2016;285:61–71.
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6.
Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584.
Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7.
Douglas GM, Maffei VJ, Zaneveld JR, Yurgel SN, Brown JR, Taylor CM, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685–8.
Antunes F, Andrade F, Araújo F, Ferreira D, Sarmento B. Establishment of a triple co-culture in vitro cell models to study intestinal absorption of peptide drugs. Eur J Pharm Biopharm. 2013;83:427–35.
Van Den Berge N, Ferreira N, Mikkelsen TW, Alstrup AKO, Tamgüney G, Karlsson P, et al. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain. 2021;144:1853–68.
Coskuner O, Wise-Scira O. Structures and free energy landscapes of the A53T mutant-type α-synuclein protein and impact of A53T mutation on the structures of the wild-type α-synuclein protein with dynamics. ACS Chem Neurosci. 2013;4:1101–13.
Yemula N, Dietrich C, Dostal V, Hornberger M. Parkinson’s disease and the gut: symptoms, nutrition, and microbiota. J Parkinsons Dis. 2021;11:1491–505.
Olanow CW, Brundin P. Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord. 2013;28:31–40.
Wittung-Stafshede P. Gut power: modulation of human amyloid formation by amyloidogenic proteins in the gastrointestinal tract. Curr Opin Struct Biol. 2022;72:33–8.
Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–85.
Lee SB, Park SM, Ahn KJ, Chung KC, Paik SR, Kim J. Identification of the amino acid sequence motif of α-synuclein responsible for macrophage activation. Biochem Biophys Res Commun. 2009;381:39–43.
Gorecki AM, Anyaegbu CC, Anderton RS. TLR2 and TLR4 in Parkinson’s disease pathogenesis: the environment takes a toll on the gut. Transl Neurodegener. 2021;10:47.
Friedland RP, Chapman MR. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017;13:e1006654.
Song S, Liu J, Zhang F, Hong JS. Norepinephrine depleting toxin DSP-4 and LPS alter gut microbiota and induce neurotoxicity in α-synuclein mutant mice. Sci Rep. 2020;10:15054.
Ahn EH, Liu X, Alam AM, Kang SS, Ye K. Helicobacter hepaticus augmentation triggers dopaminergic degeneration and motor disorders in mice with Parkinson’s disease. Mol Psychiatry. 2023;28:1337–50.
Xie Z, Zhang M, Luo Y, Jin D, Guo X, Yang W, et al. Healthy human fecal microbiota transplantation into mice attenuates MPTP-induced neurotoxicity via AMPK/SOD2 pathway. Aging Dis. 2023. https://doi.org/10.14336/AD.2023.0309.
Wallen ZD, Demirkan A, Twa G, Cohen G, Dean MN, Standaert DG, et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun. 2022;13:6958.
Gerhardt S, Mohajeri M. Changes of colonic bacterial composition in parkinson’s disease and other neurodegenerative diseases. Nutrients. 2018;10:708.
Zhang Y, He X, Qian Y, Xu S, Mo C, Yan Z, et al. Plasma branched-chain and aromatic amino acids correlate with the gut microbiota and severity of Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:48.
Shen T, Yue Y, He T, Huang C, Qu B, Lv W, et al. The association between the gut microbiota and parkinson’s disease, a meta-analysis. Front Aging Neurosci. 2021;13:636545.
Nishiwaki H, Hamaguchi T, Ito M, Ishida T, Maeda T, Kashihara K, et al. Short-chain fatty acid-producing gut microbiota is decreased in parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. mSystems. 2020;5:e00797–20.
Baldini F, Hertel J, Sandt E, Thinnes CC, Neuberger-Castillo L, Pavelka L, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18:62.
Emin D, Zhang YP, Lobanova E, Miller A, Li X, Xia Z, et al. Small soluble α-synuclein aggregates are the toxic species in Parkinson’s disease. Nat Commun. 2022;13:5512.
Acknowledgements
This study was supported by grants from the National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program”, China (2018ZX09711002–003–013), and the Scientific Innovation Project of the Chinese Academy of Sciences (XDA12040304 and XDA12040216). This project was supported by the Shanghai Committee of Science and Technology, China (18DZ2290200), and the grants from National Natural Science Foundation of China (No. 82104140, 32171220).
Author information
Authors and Affiliations
Contributions
LYF, ZXY, and YZ designed the experiments. ZXY and YZ performed the experiments. QW, LZ, YFL, and CZ performed some of the experiments. YZ, YR, and HWG assisted with the experiments. ZXY and YZ wrote the manuscript. LYF, NXZ and YZ supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Zx., Zhang, Y., Wang, Q. et al. Addition of α-synuclein aggregates to the intestinal environment recapitulates Parkinsonian symptoms in model systems. Acta Pharmacol Sin 45, 36–51 (2024). https://doi.org/10.1038/s41401-023-01150-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01150-2